Age-Related Clinical Outcomes of Patients with Non-Valvular Atrial Fibrillation: Insights from the COOL-AF Registry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
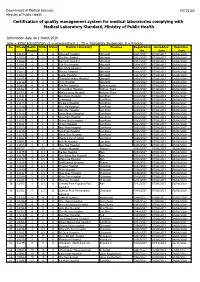
Certification of Quality Management System for Medical Laboratories Complying with Medical Laboratory Standard, Ministry of Public Health
Department of Medical Sciences F0715102 Ministry of Public Health Certification of quality management system for medical laboratories complying with Medical Laboratory Standard, Ministry of Public Health Information date on 1 March 2019 new = initial accreditation, r1 = reassessment 1 , TS = Temporary Suspension, P = Process No. HCode Health RMSc Status Medical Laboratory Province Registration Accredited Expiration Region No. Date Date 1 10673 2 2 r1 Uttaradit Hospital Uttaradit 0001/2557 07/08/2017 06/08/2020 2 11159 2 2 r1 Tha Pla Hospital Uttaradit 0002/2557 07/08/2017 06/08/2020 3 11160 2 2 r1 Nam Pat Hospital Uttaradit 0003/2557 07/08/2017 06/08/2020 4 11161 2 2 r1 Fak Tha Hospital Uttaradit 0004/2557 07/08/2017 06/08/2020 5 11162 2 2 r1 Ban Khok Hospital Uttaradit 0005/2557 07/08/2017 06/08/2020 6 11163 2 2 r1 Phichai Hospital Uttaradit 0006/2557 07/08/2017 06/08/2020 7 11164 2 2 r1 Laplae Hospital Uttaradit 0007/2557 07/08/2017 06/08/2020 8 11165 2 2 r1 ThongSaenKhan Hospital Uttaradit 0008/2557 07/08/2017 06/08/2020 9 11158 2 2 r1 Tron Hospital Uttaradit 0009/2557 07/08/2017 06/08/2020 10 10863 4 4 r1 Pak Phli Hospital Nakhonnayok 0010/2557 07/08/2017 06/08/2020 11 10762 4 4 r1 Thanyaburi Hospital Pathum Thani 0011/2557 07/08/2017 06/08/2020 12 10761 4 4 r1 Klong Luang Hospital Pathum Thani 0012/2557 07/08/2017 06/08/2020 13 11141 1 1 P Ban Hong Hospital LamPhun 0014/2557 07/08/2014 06/08/2017 14 11142 1 1 P Li Hospital LamPhun 0015/2557 07/08/2014 06/08/2017 15 11144 1 1 P Pa Sang Hospital LamPhun 0016/2557 07/08/2014 06/08/2017 -

Estimating the Burden of Α-Thalassaemia in Thailand Using A
bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Estimating the burden of α-thalassaemia in Thailand using a 2 comprehensive prevalence database for Southeast Asia 3 Carinna Hockham1,2*, Supachai Ekwattanakit3, Samir Bhatt4, Bridget S Penman5, 4 Sunetra Gupta2, Vip Viprakasit3,6 & Frédéric B Piel7 5 6 1The George Institute for Global Health, Sydney, Australia; 2 Evolutionary Ecology of 7 Infectious Disease Group, Department of Zoology, University of Oxford, Oxford, UK, 8 3Thalassaemia Centre, Faculty of Medicine, Siriraj Hospital, Mahidol University, 9 Bangkok, Thailand; 4Department of Infectious Disease Epidemiology, School of Public 10 Health, Imperial College, London, UK; 5Warwick Infectious Disease Epidemiology 11 Research, School of Life Sciences, Warwick University, Coventry, UK; 6Department of 12 Paediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, 13 Thailand; 7MRC-PHE Centre for Environment & Health, Department of Epidemiology 14 & Biostatistics, School of Public Health, Imperial College London, London, UK 15 16 *For correspondence: [email protected] 17 18 bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 Severe forms of α-thalassaemia, haemoglobin H disease and haemoglobin Bart’s hydrops 21 fetalis, are an important public health concern in Southeast Asia. -

TISCO Bank Public Company Limited Annual Report 2012
TISCO Bank Public Company Limited Annual Report 2012 Table of Contents Page Report from the Board of Directors A-1 Part 1 The Company 1. General Information 1-1 2. Risk Factors 2-1 3. Overview of TISCO Business 3-1 4. Business Operations by Area 4-1 5. Operating Assets 5-1 6. Legal Disputes 6-1 7. Capital Structure 7-1 8. Management 8-1 9. Internal Controls 9-1 10. Related Party Transactions 10-1 11. Financial Status and Performance 11-1 12. Other Related Information 12-1 Part 2 Attachment Attachment 1 Details of Directors, Management and Controlling Persons A 1-1 Attachment 2 Changes in TISCO Bank Shareholdings by Directors and Management A 2-1 Attachment 3 Report of the Audit Committee A 3-1 Attachment 4 Evaluation of the Sufficiency of Internal Control System A 4-1 Attachment 5 Statement of the Board of Directors’ Responsibility for Financial Statements A 5-1 and Auditor’s Report and Financial Statements Report from the Board of Directors In year 2012, Thai economy recovered from the aftermath of flood crisis to solid economic expansion driven by domestic consumption, following the accommodative fiscal and monetary policy, with high level of resiliency to external uncertainty. Strong economy coupled with the government stimulus programs helped to spur nationwide spending as evidenced by strong growth in private consumption and investment. Meanwhile, the Bank of Thailand eased monetary stance by lowering the policy rates to cushion against potential deterioration in export-related sectors amidst the fragile global economy. Capital market has been particularly benefited from these fundamentals as seen in the benchmark SET index rose by 36%, the best performing in Asia, whereas Bank’s credit growth has enjoyed another strong year with a growth rate of as high as 14%. -

Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveilla
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” View metadata, citation and similar papers at core.ac.uk brought to you by CORE Open Forum Infectious Diseases provided by Apollo MAJOR ARTICLE Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System Viriya Hantrakun,1, Somkid Kongyu,2 Preeyarach Klaytong,1 Sittikorn Rongsumlee,1 Nicholas P. J. Day,1,3 Sharon J. Peacock,4 Soawapak Hinjoy,2,5 and Direk Limmathurotsakul1,3,6, 1Mahidol-Oxford Tropical Medicine Research Unit (MORU), Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, 2 Epidemiology Division, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, 3 Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, Old Road Campus, University of Oxford, Oxford, United Kingdom, 4 Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 5 Office of International Cooperation, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, and 6 Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Background. National notifiable diseases surveillance system (NNDSS) data in developing countries are usually incomplete, yet the total number of fatal cases reported is commonly used in national priority-setting. Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is largely underrecognized by policy-makers due to the underreporting of fatal cases via the NNDSS. Methods. Collaborating with the Epidemiology Division (ED), Ministry of Public Health (MoPH), we conducted a retrospec- tive study to determine the incidence and mortality of melioidosis cases already identified by clinical microbiology laboratories nationwide. A case of melioidosis was defined as a patient with any clinical specimen culture positive for B. -

Mortality Risk and Temporal Patterns of Atrial Fibrillation in the Nationwide
medRxiv preprint doi: https://doi.org/10.1101/2021.01.30.21250715; this version posted June 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Mortality Risk and Temporal Patterns of Atrial Fibrillation in the Nationwide Registry Apiyasawat Short Title: Mortality Risk in Non-paroxysmal AF Sirin Apiyasawat, MD1; Sakaorat Kornbongkotmas, MD2; Ply Chichareon, MD3; Rungroj Krittayaphong, MD4; for the COOL-AF Investigators Links to Affiliations: 1 Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 2 Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand 3 Faculty of Medicine, Prince of Songkla University, Songkla, Thailand 4 Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Address for correspondence: Rungroj Krittayaphong, MD Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand Phone: (66) 2-419-6104; Fax: (66) 2-412-7412, E-mail: [email protected] Word Count: 2299 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2021.01.30.21250715; this version posted June 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . -

Saturday 5 September 2015
SATURDAY 5 SEPTEMBER 2015 SATURDAY 5 SEPTEMBER Registration Desk 0745-1730 Registration Desk, SECC for Pre-conference Workshop and Course Participants Location: Hall 4, SECC Group Meeting 1000-1700 AMEE Executive Committee Meeting (Closed Meeting) Location: Green Room 10, Back of Hall 4 AMEE-Essential Skills in Medical Education (ESME) Courses Pre-registration is essential and lunch will be provided. 0830-1700 ESME – Essential Skills in Medical Education Location: Argyll I, Crowne Plaza 0845-1630 ESMEA – Essential Skills in Medical Education Assessment Location: Argyll III, Crowne Plaza 0845-1630 RESME – Research Essential Skills in Medical Education Location: Argyll II, Crowne Plaza 0845-1700 ESMESim - Essential Skills in Simulation-based Healthcare Instruction Location: Castle II, Crowne Plaza 0900-1700 ESCEPD – Essential Skills in Continuing Education and Professional Development Location: Castle 1, Crowne Plaza 1000-1330 ESCEL – Essential Skills in Computer-Enhanced Learning Location: Carron 2, SECC Course Pre-registration is essential and lunch will be provided. 0830-1630 ASME-FLAME - Fundamentals of Leadership and Management in Education Location: Castle III, Crowne Plaza Masterclass Pre-registration is essential and lunch will be provided. 0915-1630 MC1 Communication Matters: Demystifying simulation design, debriefing and facilitation practice Kerry Knickle (Standardized Patient Program, Faculty of Medicine, University of Toronto, Canada); Nancy McNaughton, Diana Tabak) University of Toronto, Centre for Research in Education, Standardized -

Course Description of Bachelor of Science in Paramedicine 1. General Education 1.1 Social Sciences and Humanities MUGE 101 Gener
Course Description of Bachelor of Science in Paramedicine 1. General Education 1.1 Social Sciences and Humanities MUGE 101 General Education for Human Development Pre-requisite - Well-rounded graduates, key issues affecting society and the environment with respect to one’ particular context; holistically integrated knowledge to identify the key factors; speaking and writing to target audiences with respect to objectives; being accountable, respecting different opinions, a leader or a member of a team and work as a team to come up with a systematic basic research-based solution or guidelines to manage the key issues; mindful and intellectual assessment of both positive and negative impacts of the key issues in order to happily live with society and nature SHHU 125 Professional Code of Ethics Pre-requisite - Concepts and ethical principles of people in various professions, journalists, politicians, businessmen, doctors, government officials, policemen, soldiers; ethical problems in the professions and the ways to resolve them SHSS 144 Principles of Communication Pre-requisite - The importance of communication; information transferring, understanding, language usage, behavior of sender and receiver personality and communication; effective communication problems in communication SHSS 250 Public Health Laws and Regulations Pre-requisite - Introduction to law justice procedure; law and regulation for doctor and public health practitioners, medical treatment act, practice of the art of healing act, medical service act, food act, drug act, criminal -

Chapter 6 the Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network Under the Royal Patronage 6
Chapter 6 The Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network under the Royal Patronage 6 Thrathip Kolatat, Chantima Charastong At present, Thai Maternal and Child Health Network Board of Committee under the Royal Patronage has established project purposes that meet the principle objective, which is to lower the rate of preterm births. However, the board’s reexamination of the issue reveals the aforementioned strategy can be elevated to be policy-level strategy, the process of which includes setting up the clear strategic targets and public services, as well as considering the differences between service areas. It has also been suggested that personnel in those areas should be the ones coming up with action plans, to successfully reach the ultimate outcome1. Having studied the fundamental patterns of Thai Maternal and Child Health Network’s project management according to national context, the board has established an expansion model, which foresees the project expanding into various other areas, being carried out in a direction towards the expected outcome. Establishing a strategy map in each area begins with strengthening work forces in the provincial level. Since the public health system is directed by Office of Provincial Chief Medical Officer, which provides management and supports to community and district health promotion hospitals, not the general and regional hospitals. Thus, if there is to be an integration of both health promotion and treatment, an operational conference is essential. The conference would allow idea sharing and discussion between the multidisciplinary involved, namely obstetricians, pediatricians, general practitioners, registered nurses (from prenatal clinics, delivery rooms, emergency rooms, neonatal intensive care unit, neonatal wards and follow-up clinics, etc.), as well as public health technical officers, social medicine officers from community hospitals, general hospitals and regional hospitals. -

Outcome Disparities by Insurance Plan and Educational Attainment In
medRxiv preprint doi: https://doi.org/10.1101/2021.04.25.21256068; this version posted April 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Outcome Disparities by Insurance Plan and Educational Attainment in Patients with Atrial Fibrillation Apiyasawat Short Title: Education Disparities and AF Outcomes Sirin Apiyasawat, MD1; Tomon Thongsri, MD2, Kulyot Jongpiputvanich, MD3, Rungroj Krittayaphong, MD4; for the COOL-AF Investigators 1 Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 2 Buddhachinaraj Hospital, Phitsanulok, Thailand 3 Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand 4 Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Address for correspondence: Rungroj Krittayaphong, MD Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand Phone: (66) 2-419-6104; Fax: (66) 2-412-7412, E-mail: [email protected] Word Counts: 2990 Keywords: atrial fibrillation, health insurance, education, mortality, registry Apiyasawat,NOTE: This preprint et al. reports new research that has not been certified1 by peer review Educationand should not Disparities be used to guide and clinical AF Outcomes practice. medRxiv preprint doi: https://doi.org/10.1101/2021.04.25.21256068; this version posted April 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

Joint Intra-Action Review of the Public Health Response to COVID-19 in Thailand 20-24 July 2020
Joint Intra-Action Review of the Public Health Response to COVID-19 in Thailand 20-24 July 2020 Preface The Coronavirus disease 2019 (COVID-19) pandemic has posed a serious threat to health security, impacting every population and economy at the national, regional and global level. Countries have acknowledged that controlling the disease is a priority for returning to normalcy. Measures were implemented to mitigate impact and prevent health problems, as well as ensuring that everyone has access to essential health care. It is a great opportunity that the Ministry of Public Health and the World Health Organization (WHO) participated with other stakeholders in this review focusing on the implementation of COVID-19 prevention and control measures. Reviewers from the World Health Organization (WHO), international organizations, and institutes in Thailand participated in a joint review focusing on the 9 pillars of the national COVID-19 pandemic response including 1) Country-level Coordination, Planning and Monitoring, 2) Risk Communication and Community Engagement, 3) Surveillance, Rapid Response Teams, Case Investigation, 4) Points of Entry and Migrant Health, 5) National Laboratory Systems, 6) Infection Prevention and Control in the Community and Healthcare Facilities, 7) Clinical Management, 8) Operational Support and Logistics in Supply Chain and Workforce Management, and 9) Maintaining Essential Services during the COVID-19 Outbreak. Thailand was praised by the review team for its effective and successful prevention and control of COVID-19 -

วารสารวิจัยและนวัตกรรมทางสุขภาพ Journal of Health Research and Innovation
วิทยาลัยพยาบาลบรมราชชนนี สุราษฎร์ธานี The Conducting of Self-Help Group in Adolescents with Cancer: Nurses’ Roles วารสารวิจัยและนวัตกรรมทางสุขภาพ Journal of Health Research and Innovation ปี ที ่ 3 ฉบับที ่ 2 กรกฎาคม – ธันวาคม 2563 วัตถุประสงค์ 1. เพื่อเผยแพร่ผลงานวิจัย บทความทางวิชาการและนวัตกรรมทางสุขภาพของอาจารย์ บุคลากร นักศึกษา วิทยาลัยพยาบาลบรมราชชนนี สุราษฎร์ธานี ในด้านการแพทย์ การพยาบาล การสาธารณสุข การศึกษาใน สาขาวิทยาศาสตร์สุขภาพ และด้านอื่น ๆ ที่เกี่ยวข้องกับวิทยาศาสตร์สุขภาพ 2. เพื่อเผยแพร่ผลงานวิจัย บทความทางวิชาการและนวัตกรรมทางสุขภาพของบุคลากรทางการแพทย์ นักวิชาการผู้ปฏิบัติงานในศาสตร์ที่เกี่ยวข้องตลอดจนศิษย์เก่า และผู้สนใจ ในด้านการแพทย์ การพยาบาล การสาธารณสุข การศึกษาในสาขาวิทยาศาสตร์สุขภาพ และด้านอื่น ๆ ที่เกี่ยวข้องกับวิทยาศาสตร์สุขภาพ 3. เพื่อสร้างเครือข่ายทางวิชาการทั้งในวิทยาลัยพยาบาลบรมราชชนนี สุราษฎร์ธานี และสถาบันวิชาชีพ ที่เกี่ยวข้อง 4. เพื่อตอบสนองพันธกิจหลักในการสร้างองค์ความรู้และการเผยแพร่ผลงานวิชาการและงานวิจัยของ วิทยาลัยพยาบาลบรมราชชนนี สุราษฎร์ธานี สำนักงำน บรรณาธิการวารสาร วิทยาลัยพยาบาลบรมราชชนนี สุราษฎร์ธานี 56/6 หมู่ 2 ถ.ศรีวิชัย ตาบล มะขามเตี้ย อาเภอเมือง จังหวัดสุราษฎร์ธานี 84000 โทร. 0-7728-7816 ต่อ 218 โทรสาร 0-7727-2571 http://www.bcnsurat.ac.th E-mail: [email protected] พมิ พท์ ี่ โรงพิมพ์เลิศไชย 16/4-6 ถนนไตรอนสุ นธิ์ ตาบลตลาด อาเภอเมืองสุราษฎร์ธานี จังหวัดสุราษฎร์ธานี โทรศัพท์ 0 7727 3973 โทรสาร 0 7729 9521 วารสารวจิ ยั และนวตั กรรมทางสขุ ภาพ เป็นวารสารทมี่ ผี ูท้ รงคณุ วฒุ ติ รวจสอบเนอื้ หาบทความเพอื่ ลงตพี มิ พจ์ า นวน 2 ท่านต่อบทความ และ บทความหรอื ขอ้ คดิ เหน็ ใด ๆ ทปี่ รากฏในวารสารวจิ -

The Thai Journal of SURGERY
ISSN 0125-6068 E-ISSN 2697-5858 The Thai Journal of SURGERY Official Publication of The Royal College of Surgeons of Thailand www.tci-thaijo.org/index.php/ThaiJSurg/index Volume 41 July-September 2020 Number 3 REVIEW ARTICLE 65 Nipple Sparing Mastectomy in Breast Cancer Patients Chayanoot Rattadilok, Prakasit Chirappapha, Prapa Rattadilok ORIGINAL ARTICLE 79 Simplified ERAS Program for Elective Colorectal Surgery at Suratthani Hospital Sorawat Janwanitchsthaporn, Jirapong Intarasompun CASE REPORTS 84 A Report of Three Patients Surviving Near-Fatal Blunt Cardiac Rupture Komkrit Komuttarin, Nisit Poolthananant, Araya Thitisurawat, Witchayoot Teerapinyo 92 Klebsiella Distinctive Syndrome Presenting with Muscular Abscess and Osteomyelitis Nutthawut Akaranuchat, Chanon Parkpinyo ABSTRACTS 96 Abstracts of the 45th Annual Scientific Congress of The Royal College of Surgeons of Thailand, 8-11 October 2020, RCST Virtual Annual Congress 2020 (Part I) Secretariat Office : Royal Golden Jubilee Building, 2 Soi Soonvijai, New Petchaburi Road, Huaykwang, Bangkok 10310, Thailand Tel. +66 2716 6141-3 Fax +66 2716 6144 E-mail: [email protected] www.tci-thaijo.org/index.php/ThaiJSurg/index Royal College of Surgeons of Thailand Secretariat Office : Royal Golden Jubilee Building, 2 Soi Soonvijai, New Petchburi Road, Bangkok 10310, Thailand OFFICERS 2019 - 2021 President : Paisit Siriwittayakorn President-Elect : Pramook Mutirangura Vice President : Charnvate Satthaputh Secretary General : Sutdhachit Linananda Assistant Scretary : Pornprom Muangman Treasurer