Estramustine Depolymerizes Microtnbules by Binding to Tubulin 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
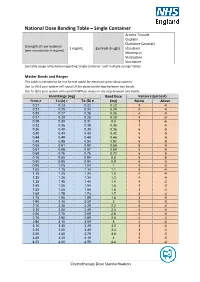
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -
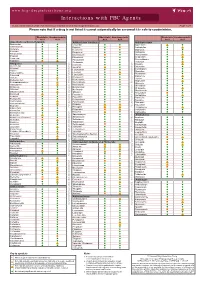
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage. -

Confidential: for Review Only
BMJ Confidential: For Review Only The risk of fall and fracture with the initiation of a prostate - selective alpha antagonist Journal: BMJ Manuscript ID: BMJ.2015.028205 Article Type: Research BMJ Journal: BMJ Date Submitted by the Author: 17-Jul-2015 Complete List of Authors: Welk, Blayne; Western University, McArthur, Eric; Institute for Clinical Evaluative Sciences,, Fraser, Lisa-Ann; Western University, Medicine Hayward, Jade; Institute for Clinical Evaluative Sciences,, Dixon, Stephanie; Institute for Clinical Evaluative Sciences,, Hwang, Joseph; Case Western Reserve University School of Medicine, Ordon, Michael; University of Toronto, Surgery (Urology) Keywords: BPH, Fall, Fracture, Alpha antgonist https://mc.manuscriptcentral.com/bmj Page 1 of 45 BMJ 1 2 The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist 3 4 1,2,3 2 4 2 5 Blayne Welk MD MSc , Eric McArthur MSc , Lisa-Ann Fraser MD MSc , Jade Hayward , 6 Stephanie Dixon MSc PhD 2,3 , Y. Joseph Hwang MSc 5, Michael Ordon MD MSc 6 7 8 1 DepartmentConfidential: of Surgery, Western University, For London Review, Ontario, Canada Only 9 2 Institute for Clinical Evaluative Sciences, London, Ontario, Canada 10 11 3 Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada 12 4 Department of Medicine, Western University, London, Ontario 13 5 MD Candidate, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA 14 6 Division of Urology, Department of Surgery, University of Toronto, Toronto, Ontario, Canada 15 16 17 Correspondence: 18 Blayne Welk, MD MSc 19 Assistant Professor, Division of Urology and Epidemiology and Biostatistics 20 Western University 21 Room B4-667 22 23 St Joseph's Health Care 24 268 Grosvenor Street London ON N6A 4V2 25 Telephone: 519 646-6367 | Fax: 519 646-6037 26 [email protected] 27 28 29 Addresses: 30 Mr McArthur: [email protected] 31 LHSC – Victoria Hospital 32 ELL-101, 800 Commissioners Rd. -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Eliminating Vincristine Administration Events
Issue 37 October 2017 Eliminating vincristine administration events Issue: Despite the usually deadly consequences of accidentally administering the chemotherapy drug vincristine intrathecally, adverse events still occur, typically because some organizations still administer vincristine via syringe. The good news is that these events are happening less in the United States, mostly because of the efforts of leading national organizations to promote an effective prevention strategy that assures a mechanical barrier to intrathecal administration (into the subarachnoid space). The strategy involves diluting intravenous vincristine or other vinca alkaloids in a minibag that contains a volume that is too large for intrathecal administration (e.g., 25 mL for pediatric patients and 50 mL for adults), making it mechanically difficult to accidentally administer intrathecally.1 Vinca alkaloids (vinblastine, vinorelbine, vincristine, and vincristine liposomal) are chemotherapy drugs that are intended to be administered intravenously. If given intrathecally, vincristine is nearly always fatal and associated with an irreversible, painful ascending paralysis.2 When vinca alkaloids are injected intrathecally, destruction of the central nervous system occurs, radiating out from the injection site. The few survivors of this adverse event experienced devastating neurological damage.1 Part of the problem stems from ordering intravenous vincristine in conjunction with medications that are administered intrathecally via a syringe, such as methotrexate, cytarabine and hydrocortisone. In some adverse events, vincristine was mistakenly injected into the cerebrospinal fluid (CSF) of patients when the intent was to inject another intrathecal chemotherapy agent, such as methotrexate or cytarabine.3 Intravenous vincristine and other vinca alkaloids are dispensed from the pharmacy with explicit warning labels about their lethality if given intrathecally. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Antimicrotubule Effects of Estramustine, an Antiprostatic Tumor Drug
[CANCER RESEARCH 45, 3891-3897, August 1985] Antimicrotubule Effects of Estramustine, an Antiprostatic Tumor Drug Mark E. Stearns1 and Kenneth D. Tew2 Departments of Anatomy [M. E. S.] and of Medicine and Pharmacology [K. D, T.], Vincent T. Lombardi Cancer Research Center, Georgetown University Hospital, Washington, DC 20007 ABSTRACT cytomatrix effects of EM and learn how cytoplasmic related effects of EM might ultimately produce the reported antimitotic Estramustine [170-estradiol 3 N bis(2-chloroethyl)carbamate; events in dividing cells (6). EM] is a stable conjugate of estradiol and nor-nitrogen mustard In this paper, we have investigated possible cytotoxic effects that is used for the treatment of human prostatic carcinoma. We of EM at the cytological level. For these studies, the fish erythro- have studied the cytotoxic effects of EM on the cytoskeletal phore or red pigment cell has been used as a model system for organization of squirrelfish pigment cells (erythrophores) and investigation of the cytotoxic consequences of EM. There are a human prostatic tumor cells (DU 145) in culture. Light and whole- number of attractive reasons for utilizing erythrophores for the mount electron microscopy studies reveal that, at /¿Mlevels(60 work described here. Erythrophores are symmetrical cells with to 120 ¿IM),EMhas a dose-dependent disruptive effect on cell thousands of radially ordered microtubules which control the shape, cytoskeletal organization, and intracellular transport. directed motion of numerous red pigment granules (14, 19). At Upon removal of the drug, the cytological effects of EM are the light microscopic level, the pigment is observed to pulsate or rapidly reversible in fish cells but not DU 145s. -

Order in Council 1243/1995
PROVINCE OF BRITISH COLUMBIA ORDER OF THE LIEUTENANT GOVERNOR IN COUNCIL Order in Council No. 12 4 3 , Approved and Ordered OCT 121995 Lieutenant Governor Executive Council Chambers, Victoria On the recommendation of the undersigned, the Lieutenant Governor, by and with the advice and consent of the Executive Council, orders that Order in Council 1039 made August 17, 1995, is rescinded. 2. The Drug Schedules made by regulation of the Council of the College of Pharmacists of British Columbia, as set out in the attached resolution dated September 6, 1995, are hereby approved. (----, c" g/J1"----c- 4- Minister of Heal fandand Minister Responsible for Seniors Presidin Member of the Executive Council (This pan is for adnwustratlye purposes only and is not part of the Order) Authority under which Order Is made: Act and section:- Pharmacists, Pharmacy Operations and Drug Scheduling Act, section 59(2)(1), 62 Other (specify): - Uppodukoic1enact N6145; Resolution of the Council of the College of Pharmacists of British Columbia ("the Council"), made by teleconference at Vancouver, British Columbia, the 6th day of September 1995. RESOLVED THAT: In accordance with the authority established in Section 62 of the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia, S.B.C. Chapter 62, the Council makes the Drug Schedules by regulation as set out in the attached schedule, subject to the approval of the Lieutenant Governor in Council. Certified a true copy Linda J. Lytle, Phr.) Registrar DRUG SCHEDULES to the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia The Drug Schedules have been printed in an alphabetical format to simplify the process of locating each individual drug entry and determining its status in British Columbia.