Combination Chemotherapy with Estramustine Phosphate, Ifosfamide and Cisplatin for Hormone-Refractory Prostate Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIP - Ifosfamide, Cisplatin and Etoposide
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST Systemic Anti Cancer Treatment Protocol VIP - Ifosfamide, Cisplatin and Etoposide PROTOCOL REF: MPHA VIPGC (Version No: 1.1) Approved for use in: Germ cell Dosage: Drug Dosage Route Frequency Etoposide 75mg/m2 days 1 to 5 IV Every 21 days Cisplatin 20mg/m2 days 1 to 5 IV Every 21 days Mesna 200mg/m2 days 1 to 5 IV Every 21 days Ifosfamide 1500mg/m2 + 1500mg/m2 IV Every 21 days +Mesna days 1 to 5 Mesna 1200mg 1 to 5 Oral Every 21 days Supportive treatments: Domperidone 10mg oral tablets, up to 3 times a day or as required Dexamethasone tablets, 4mg twice daily for 3 days Filgrastim 30MU or 48MU subcutaneous injection daily for 7 days starting on day 6, repeat FBC and continue for further 7 days if neutrophil count has not recovered to 1.0 x 109/L Extravasation risk: Etoposide – Irritant Cisplatin – Exfoliant Ifosfamide - Neutral Issue Date: 31st January 2019 Review Date: January 2022 Page 1 of 10 Protocol reference: MPHAVIPGC Author: Nick Armitage Authorised by: Dr. Ali Version No:1.1 THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST Administration: Day Drug Dosage Route Diluent and Rate 1 Dexamethasone 8mg PO 30 mins before chemotherapy 1 Ondansetron 16mg PO 30 mins before chemotherapy 1 Etoposide 75mg/m2 IV In 250 to 1000mL sodium chloride 0.9% over 60 minutes 1 Cisplatin 20mg/m2 IV 1000mL 0.9% sodium chloride over 90 minutes 1 Mesna 200mg/m2 IV In 500mL sodium chloride 0.9% over 15 minutes 1 Ifosfamide + Mesna 1500mg/m2 IV In 1000mL sodium chloride + 0.9% over 4 hours 1500mg/m2 -

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
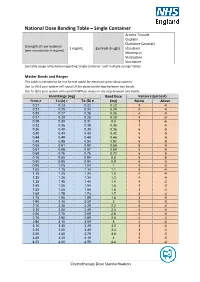
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

The Temperature-Dependent Effectiveness of Platinum-Based
cells Article The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines Roxan F.C.P.A. Helderman 1,2 , Daan R. Löke 2, Jan Verhoeff 3 , Hans M. Rodermond 1, Gregor G.W. van Bochove 1, Menno Boon 1, Sanne van Kesteren 1, Juan J. Garcia Vallejo 3, H. Petra Kok 2, Pieter J. Tanis 4 , Nicolaas A.P. Franken 1,2 , Johannes Crezee 2 and Arlene L. Oei 1,2,* 1 Laboratory for Experimental Oncology and Radiobiology (LEXOR), Center for Experimental and Molecular Medicine (CEMM), Amsterdam University Medical Centers (UMC), University of Amsterdam, Cancer Center Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (R.F.C.P.A.H.); [email protected] (H.M.R.); [email protected] (G.G.W.v.B.); [email protected] (M.B.); [email protected] (S.v.K.); [email protected] (N.A.P.F.) 2 Department of Radiation Oncology, Amsterdam UMC, University of Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (D.R.L.); [email protected] (H.P.K.); [email protected] (J.C.) 3 Department of Molecular Cell Biology & Immunology, Amsterdam Infection & Immunity Institute and Cancer Center Amsterdam, Amsterdam UMC, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands; j.verhoeff@amsterdamumc.nl (J.V.); [email protected] (J.J.G.V.) 4 Department for Surgery, Amsterdam UMC, University of Amsterdam, Cancer Center Amsterdam, P.O. -

August 2019: Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 39TH EDITION Cumulative Supplement Number 08 : August 2019 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; BENZHYDROCODONE HYDROCHLORIDE TABLET;ORAL APADAZ >D> + KVK TECH INC 325MG;EQ 8.16MG BASE N 208653 003 Jan 04, 2019 Aug CHRS >A> +! 325MG;EQ 8.16MG BASE N 208653 003 Jan 04, 2019 Aug CHRS ACETAMINOPHEN; CODEINE PHOSPHATE TABLET;ORAL ACETAMINOPHEN AND CODEINE PHOSPHATE >A> AA ELITE LABS INC 300MG;15MG A 212418 001 Sep 10, 2019 Aug NEWA >A> AA 300MG;30MG A 212418 002 Sep 10, 2019 Aug NEWA >A> AA 300MG;60MG A 212418 003 Sep 10, 2019 Aug NEWA ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE TABLET;ORAL OXYCODONE AND ACETAMINOPHEN >D> AA CHEMO RESEARCH SL 325MG;5MG A 207574 001 Dec 13, 2016 Aug CAHN >A> AA HALO PHARM CANADA 325MG;5MG A 207834 001 Aug 15, 2019 Aug NEWA >A> AA 325MG;7.5MG A 207834 002 Aug 15, 2019 Aug NEWA >A> AA 325MG;10MG A 207834 003 Aug 15, 2019 Aug NEWA >A> AA XIROMED 325MG;5MG A 207574 001 Dec 13, 2016 Aug CAHN ACYCLOVIR CAPSULE;ORAL ACYCLOVIR >A> AB CADILA 200MG A 204313 001 Mar 25, 2016 Aug CAHN >D> AB ZYDUS PHARMS 200MG A 204313 001 Mar 25, 2016 Aug CAHN >D> OINTMENT;OPHTHALMIC >D> AVACLYR >D> +! FERA PHARMS LLC 3% N 202408 001 Mar 29, 2019 Aug DISC >A> + @ 3% N 202408 001 Mar 29, 2019 Aug DISC OINTMENT;TOPICAL ACYCLOVIR >A> AB APOTEX INC 5% A 210774 001 Sep 06, 2019 Aug NEWA >D> AB PERRIGO UK FINCO 5% A 205659 001 Feb 20, 2019 Aug DISC >A> @ 5% A 205659 001 Feb 20, 2019 Aug DISC ALBENDAZOLE TABLET;ORAL ALBENDAZOLE >A> AB STRIDES PHARMA 200MG A 210011 -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

Induction with Mitomycin C, Doxorubicin, Cisplatin And
British Journal of Cancer (1999) 80(12), 1962–1967 © 1999 Cancer Research Campaign Article no. bjoc.1999.0627 Induction with mitomycin C, doxorubicin, cisplatin and maintenance with weekly 5-fluorouracil, leucovorin for treatment of metastatic nasopharyngeal carcinoma: a phase II study RL Hong1, TS Sheen2, JY Ko2, MM Hsu2, CC Wang1 and LL Ting3 Departments of 1Oncology, 2Otolaryngology and 3Radiation Therapy, National Taiwan University Hospital, National Taiwan University, No. 7, Chung-Shan South Road, Taipei 10016, Taiwan Summary The combination of cisplatin and 5-fluorouracil (5-FU) (PF) is the most popular regimen for treating metastatic nasopharyngeal carcinoma (NPC) but it is limited by severe stomatitis and chronic cisplatin-related toxicity. A novel approach including induction with mitomycin C, doxorubicin and cisplatin (MAP) and subsequent maintenance with weekly 5-FU and leucovorin (FL) were designed with an aim to reduce acute and chronic toxicity of PF. Thirty-two patients of NPC with measurable metastatic lesions in the liver or lung were entered into this phase II trial. Mitomycin C 8 mg m–2, doxorubicin 40 mg m–2 and cisplatin 60 mg m–2 were given on day 1 every 3 weeks as initial induction. After either four courses or remission was achieved, patients received weekly dose of 5-FU 450 mg m–2 and leucovorin 30 mg m–2 for maintenance until disease progression. With 105 courses of MAP given, 5% were accompanied by grade 3 and 0% were accompanied by grade 4 stomatitis. The dose-limiting toxicity of MAP was myelosuppression. Forty per cent of courses had grade 3 and 13% of courses had grade 4 leukopenia. -

Docetaxel, Ifosfamide and Cisplatin (DIP) in Squamous Cell Carcinoma of the Head and Neck
ANTICANCER RESEARCH 29: 5137-5142 (2009) Docetaxel, Ifosfamide and Cisplatin (DIP) in Squamous Cell Carcinoma of the Head and Neck POL M. SPECENIER1, JAN VAN DEN BRANDE1, DIRK SCHRIJVERS1,2, MANON T. HUIZING1, SEVILAY ALTINTAS1, JOKE DYCK1, DANIELLE VAN DEN WEYNGAERT3, CARL VAN LAER4 and JAN B. VERMORKEN1 Departments of 1Medical Oncology, 3Radiotherapy and 4Otolaryngology, Antwerp University Hospital, Edegem; 2Department of Medical Oncology, ZNA Middelheim, Antwerp, Belgium Abstract. Background: Docetaxel, ifosfamide and cisplatin response, 19 partial responses, 1 stable disease); the complete have all shown activity in squamous cell carcinoma of the head response rate increased to 42% after 4 × DIP. No dose or and neck (SCCHN). The optimal combination of the three drugs sequence effect was evident. The minimum follow-up of the is, however, unknown. Considering the favorable results of surviving patients was 51 months, with median relapse-free taxane-containing triplets as induction chemotherapy in locally survival of 13.8 months and median overall survival of 18.8 advanced (LA) SCCHN, DIP (docetaxel, ifosfamide, cisplatin) months. Only four patients relapsed at distant sites. Conclusion: was studied in this setting as part of a phase I dose- and DIP is highly active in previously untreated LA SCCHN, sequence-exploring study. Patients and Methods: D (60 or 75 however, toxicity of DIP in this population is substantial. mg/m2) was given by 60-min infusion on day 1, I (1000 mg/m2/day), with mesna until 12 hours after I, by 24-h infusion For over two decades, cisplatin has been the backbone of days 1-5, and P (50 or 75 mg/m2) by 24-h infusion on days 1 or chemotherapeutic regimens which are used for the treatment 5. -

Arsenic Trioxide Is Highly Cytotoxic to Small Cell Lung Carcinoma Cells
160 Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells 1 1 Helen M. Pettersson, Alexander Pietras, effect of As2O3 on SCLC growth, as suggested by an Matilda Munksgaard Persson,1 Jenny Karlsson,1 increase in neuroendocrine markers in cultured cells. [Mol Leif Johansson,2 Maria C. Shoshan,3 Cancer Ther 2009;8(1):160–70] and Sven Pa˚hlman1 1Center for Molecular Pathology, CREATE Health and 2Division of Introduction Pathology, Department of Laboratory Medicine, Lund University, 3 Lung cancer is the most frequent cause of cancer deaths University Hospital MAS, Malmo¨, Sweden; and Department of f Oncology-Pathology, Cancer Center Karolinska, Karolinska worldwide and results in 1 million deaths each year (1). Institute and Hospital, Stockholm, Sweden Despite novel treatment strategies, the 5-year survival rate of lung cancer patients is only f15%. Small cell lung carcinoma (SCLC) accounts for 15% to 20% of all lung Abstract cancers diagnosed and is a very aggressive malignancy Small cell lung carcinoma (SCLC) is an extremely with early metastatic spread (2). Despite an initially high aggressive form of cancer and current treatment protocols rate of response to chemotherapy, which currently com- are insufficient. SCLC have neuroendocrine characteristics bines a platinum-based drug with another cytotoxic drug and show phenotypical similarities to the childhood tumor (3, 4), relapses occur in the absolute majority of SCLC neuroblastoma. As multidrug-resistant neuroblastoma patients. At relapse, the efficacy of further chemotherapy is cells are highly sensitive to arsenic trioxide (As2O3) poor and the need for alternative treatments is obvious. in vitro and in vivo, we here studied the cytotoxic effects Arsenic-containing compounds have been used in tradi- of As2O3 on SCLC cells. -

CARBOPLATIN- Carboplatin Injection Accord Healthcare, Inc
CARBOPLATIN- carboplatin injection Accord Healthcare, Inc. --------- CARBOplatin Injection Rxo nly Carboplatin injection should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available. Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug related side effect. Anaphylactic-like reactions to carboplatin have been reported and may occur within minutes of carboplatin injection administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms. DESCRIPTION Carboplatin injection is supplied as a sterile, pyrogen-free, 10 mg/mL aqueous solution of carboplatin, USP. Carboplatin, USP is a platinum coordination compound. The chemical name for carboplatin, USP is platinum, diammine [1,1-cyclobutanedicarboxylato(2-)-0,0']-,(SP-4-2), and carboplatin, USP has the following structural formula: C6H12N2O4Pt M.W. 371.25 Carboplatin, USP is a crystalline powder. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5 to 7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide. CLINICAL PHARMACOLOGY Carboplatin, like cisplatin, produces predominantly interstrand DNA cross-links rather than DNA-protein cross-links. This effect is apparently cell-cycle nonspecific. The aquation of 2 carboplatin, which is thought to produce the active species, occurs at a slower rate than in the case of cisplatin. Despite this difference, it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links, causing equivalent lesions and biological effects. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

BC Cancer Protocol Summary for Neoadjuvant Or Adjuvant Therapy for Breast Cancer Using Docetaxel, Carboplatin, and Trastuzumab
BC Cancer Protocol Summary for Neoadjuvant or Adjuvant Therapy for Breast Cancer Using DOCEtaxel, CARBOplatin, and Trastuzumab Protocol Code BRAJDCARBT Tumour Group Breast Contact Physician Dr. Susan Ellard ELIGIBILITY: . ECOG 0-1 . Node positive or high risk node negative, including patient with T1b disease (T1a still requires CAP approval) . HER-2 over-expression defined as either IHC3+, or FISH amplification ratio greater than or equal to 2 per BC Cancer central laboratory . Adequate renal and hepatic function . Adequate hematological parameters (ANC greater than 1.5 x 109/L and platelets greater than 100 x 109/L) . No signs or symptoms of cardiac disease. LVEF greater than or equal to 50%* * If the LVEF is between 45-50%, the oncologist may decide to treat based on clinical assessment EXCLUSIONS: . ECOG 2-4 . Stage IV disease (please refer to advanced regimens) . Significant hepatic dysfunction, contraindicating DOCEtaxel . Significant cardiovascular disease and/or LVEF less than 50%; if initial reading is less than 50%, physician may consider repeating for validity, or assessing LVEF by the other modality, e.g. echocardiogram instead of MUGA . greater than or equal to grade 2 sensory or motor neuropathy . Pregnancy or lactation TESTS: . Baseline: CBC & diff, platelets, bilirubin, GGT, ALT, LDH, alkaline phosphatase, creatinine, (see Precaution #5 for guidelines regarding hepatic dysfunction and DOCEtaxel), suggested: nuclear renogram for GFR (if available locally, and not previously done) . Before each treatment cycle: CBC & diff, platelets, creatinine . MUGA scan or echocardiogram: prior to first treatment with trastuzumab and every 3-4 months until completion of treatment per the discretion of the treating physician.