Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASCC/ESMO ANTIEMETIC GUIDELINE 2016 with Updates in 2019
1 ANTIEMETIC GUIDELINES: MASCC/ESMO MASCC/ESMO ANTIEMETIC GUIDELINE 2016 With Updates in 2019 Organizing and Overall Meeting Chairs: Matti Aapro, MD Richard J. Gralla, MD Jørn Herrstedt, MD, DMSci Alex Molassiotis, RN, PhD Fausto Roila, MD © Multinational Association of Supportive Care in CancerTM All rights reserved worldwide. 2 ANTIEMETIC GUIDELINES: MASCC/ESMO These slides are provided to all by the Multinational Association of Supportive Care in Cancer and can be used freely, provided no changes are made and the MASCC and ESMO logos, as well as date of the information are retained. For questions please contact: Matti Aapro at [email protected] Chair, MASCC Antiemetic Study Group or Alex Molassiotis at [email protected] Past Chair, MASCC Antiemetic Study Group 3 ANTIEMETIC GUIDELINES: MASCC/ESMO Consensus A few comments on this guideline set: • This set of guideline slides represents the latest edition of the guideline process. • This set of slides has been endorsed by the MASCC Antiemetic Guideline Committee and ESMO Guideline Committee. • The guidelines are based on the votes of the panel at the Copenhagen Consensus Conference on Antiemetic Therapy, June 2015. • Latest version: March 2016, with updates in 2019. 4 ANTIEMETIC GUIDELINES: MASCC/ESMO Changes: The Steering Committee has clarified some points: 2016: • A footnote clarified that aprepitant 165 mg is approved by regulatory authorities in some parts of the world ( although no randomised clinical trial has investigated this dose ). Thus use of aprepitant 80 mg in the delayed phase is only for those cases where aprepitant 125 mg is used on day 1. • A probable modification in pediatric guidelines based on the recent Cochrane meta-analysis is indicated. -

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
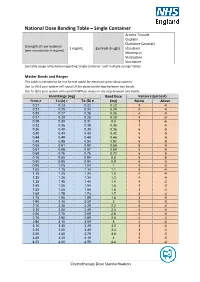
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

Combining Paclitaxel and Lapatinib As Second-Line Treatment for Patients with Metastatic Transitional Cell Carcinoma: a Case Series
ANTICANCER RESEARCH 32: 3949-3952 (2012) Combining Paclitaxel and Lapatinib as Second-line Treatment for Patients with Metastatic Transitional Cell Carcinoma: A Case Series STÉPHANE CULINE, ZINEB SELLAM, LINDA BOUAITA, ELIAS ASSAF, CATHERINE DELBALDO, MURIEL VERLINDE-CARVALHO and DAMIEN POUESSEL Department of Medical Oncology, Henri Mondor Hospital, Créteil, France Abstract. Background: Current first-line cisplatin-based trial comparing vinflunine with best supportive care (BSC) combination chemotherapy regimens provide interesting to BSC alone, an estimated difference in overall survival response rates but limited impact on survival for patients with (OS) of 2 months was reached in the intent-to-treat metastatic transitional cell carcinoma of the urothelium. Such population. However, a significant difference in OS was only results leave a significant patient population in need of salvage seen after removing patients who had major protocol therapy. Patients and Methods: As the epidermal growth factor violations (2). Therefore therapy for patients who fail first- receptors 1 and 2 (EGFR and HER2) are frequently line cisplatin-based chemotherapy remains a highly unmet overexpressed in urothelial carcinoma, we explored the medical need. feasibility of a combination of paclitaxel (80 mg/m2/week) and In a phase II study led by the French Genito-Urinary lapatinib (1,500 mg orally daily) for six patients who were Tumor group (GETUG), the activity of weekly paclitaxel as treated after failure of first-line platinum-based chemotherapy. second-line chemotherapy was assessed in 45 patients with Results: Only one out of six patients was able to receive the MTCCU. A low objective response rate (9%) along with a full doses during the first six weeks of treatment, while grade high rate of stabilization (38%) suggested limited impact as 2 or 3 diarrhea events required lapatinib dose reduction (one a single agent (3). -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

Non-Steroidal Anti-Inflammatory Drugs Inhibit Bone Healing: a Review S
Review Article © Schattauer 2010 385 Non-steroidal anti-inflammatory drugs inhibit bone healing: A review S. Barry Washington State University, Department of Veterinary Clinical Sciences, Veterinary Teaching Hospital, Pullman, Wash- ington, USA crine and autocrine activity, have since Keywords stems from prostaglandin inhibition and is been shown to regulate constitutive and in- Non-steroidal anti-inflammatory drugs, likely multifactorial. In human medicine ducible functions throughout the body, in- NSAID, bone healing NSAID are known to prevent heterotopic ossi- cluding bone healing (5–9). The mech- fication, however the clinical importance of anism of NSAID inhibition to bone healing Summary their effects on bone healing remains contro- is unknown, but is likely multifactorial. Re- The ability of non-steroidal anti-inflammatory versial. Although a small handful of reports searchers have suggested that NSAID affect drugs (NSAID) to inhibit bone healing has suggest that NSAID suppress bone healing in normal bone healing in multiple ways, with been established in experimental animal dogs and horses, there is little published infor- emphasis often (but not exclusively) placed models using mice, rats, and rabbits. The mation to direct veterinary practice in do- on processes related to the inflammatory mechanism of action is largely unknown but mestic species. stage. Deciphering the mechanism of NSAID inhibition requires an understanding of Correspondence to: Vet Comp Orthop Traumatol 2010; 23: 385–392 Sabrina Barry, DVM doi:10.3415/VCOT-10-01-0017 fracture healing. Fracture healing presents Washington State University Received: January 31, 2010 an exquisitely orchestrated series of coor- Department of Veterinary Clinical Sciences Accepted: June 23, 2010 dinated molecular and cellular events. -

Latest Administration Hour Prior to Competition Max Dosage Per Pound of Body Weight Medication Trade Name Medication Generic Name
MEDICATION MEDICATION MAX DOSAGE PER POUND LATEST ADMINISTRATION HOUR ADMINISTRATION METHOD GENERIC NAME TRADE NAME OF BODY WEIGHT PRIOR TO COMPETITION (single dose per 24 hours unless specified otherwise) Dexamethasone Azium® 2.0 mg/100Lb >12 hours IV, IM (20 mg/1000Lb) or 0.5 mg/100Lb >6 hours IV (5.0 mg/1000Lb) or 1.0 mg/100LB >6 hours Oral (10 mg/1000Lb) Diclofenac Surpass® 5 inch ribbon, 1⁄2 inch thick, >12 hours Topical, 2 doses each day 12 hours apart one site Firocoxib Equioxx® 0.1 mg/kg >12 hours Oral (0.0455 mg/Lb) (45.5 mg/1000Lb) Phenylbutazone (“bute”) * Butazolidin® 2.0 mg/Lb >12 hours Oral, IV (2.0 grams/1000Lb) or 1.0 mg/Lb AM & PM feed Oral, 2 doses each day, 12 hours apart (1.0 grams/1000Lb) Flunixin meglumine * Banamine® 0.5 mg/Lb >12 hours Oral, IV (500 mg/1000Lb) Ketoprofen Ketofen® 1.0 mg/Lb >4 hours, but IV (1.0 gram/1000Lb) >6 hours is recommended Meclofenamic acid Arquel® 0.5 mg/Lb Oral, 2 doses each day, 12 hours apart (500 mg/1000Lb) Naproxen Naprosyn® 4.0 mg/Lb >12 hours Oral (4.0 grams/1000Lb) Eltenac Not yet approved Telzenac® 0.25 mg/Lb (250 mg/1000Lb) 12 hours IV Methocarbamol Robaxin® 5.0 mg/Lb >6 hours Oral, IV, 2 doses each day, 12 hours apart (5.0 grams/1000Lb) * Do not administer phenylbutazone and flunixin at the same time (Unless used according to The maximum treatment time for any of the above permitted medication is five days, with the Section 8). -

Simultaneous Determination of Residues of Non-Steroidal Anti-Inflammatory Drugs and Glucocorticosteroids in Animal Muscle By
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Food Anal. Methods (2016) 9:1837–1848 DOI 10.1007/s12161-015-0352-y Simultaneous Determination of Residues of Non-Steroidal Anti-Inflammatory Drugs and Glucocorticosteroids in Animal Muscle by Liquid Chromatography-Tandem Mass Spectrometry Piotr Jedziniak1 & Małgorzata Olejnik1 & Konrad Pietruk1 & Edyta Protasiuk1 & Teresa Szprengier-Juszkiewicz1 & Jan Żmudzki1 Received: 11 February 2015 /Accepted: 4 November 2015 /Published online: 21 November 2015 # The Author(s) 2015. This article is published with open access at Springerlink.com Abstract A method for the determination of a wide range Introduction residues of anti-inflammatory drugs (16 acidic non-steroidal anti-inflammatory drugs and four metamizole metabolites and Non-steroidal anti-inflammatory drugs (NSAIDs) and five corticosteroids) has been was developed. In the first step glucocorticosteroids (GCs) are widely used in veterinary medi- of sample preparation, acetate buffer was added to minced cine as well as in treatment of diseases in food-producing ani- muscle samples and 15-min ultrasound-assisted enzymatic mals. Despite its effectiveness, the important drawback of phar- hydrolysis was performed. Next, the samples were extracted macotherapy is drug residues in animal tissues. It became an twice with acetonitrile, freezed and analysed. The analytes important issue in the food safety. Potential toxicity of medicinal were separated on a C18 column with a 25-min gradient of veterinary products has to be evaluated before the drug registra- methanol/acetonitrile (8:2) and 0.05 M ammonium formate at tion. When necessary, maximum residue limits (MRLs) in food pH 5.0 and determined by liquid chromatography-tandem are established. -

Non-Steroidal Anti-Inflammatory Drugs As Chemopreventive Agents: Evidence from Cancer Treatment in Domestic Animals
Annual Research & Review in Biology 26(1): 1-13, 2018; Article no.ARRB.40829 ISSN: 2347-565X, NLM ID: 101632869 Non-Steroidal Anti-Inflammatory Drugs as Chemopreventive Agents: Evidence from Cancer Treatment in Domestic Animals Bianca F. Bishop1 and Suong N. T. Ngo1* 1School of Animal and Veterinary Sciences, The University of Adelaide, Roseworthy, SA 5371, Australia. Authors’ contributions This work was carried out in collaboration between both authors. Author BFB performed the collection and analysis of the data. Author SNTN designed the study, managed the analyses and interpretation of the data and prepared the manuscript. Both authors read and approved the final manuscript. Article Information DOI: 10.9734/ARRB/2018/40829 Editor(s): (1) David E. Martin, Martin Pharma Consulting, LLC, Shawnee, OK, USA. (2) George Perry, Dean and Professor of Biology, University of Texas at San Antonio, USA. Reviewers: (1) Fulya Ustun Alkan, Istanbul University, Turkey. (2) Thompson Akinbolaji, USA. (3) Ramesh Gurunathan, Sunway Medical Center, Malaysia. (4) Mohamed Ahmed Mohamed Nagy Mohamed, El Minia Hospital, Egypt. Complete Peer review History: http://www.sciencedomain.org/review-history/24385 Received 10th February 2018 Accepted 21st April 2018 Review Article Published 30th April 2018 ABSTRACT Aims: This study aims to systematically review currently available data on the use of non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of cancer in domestic animals to evaluate the efficacy of different treatment protocols and to suggest further recommendations for future study. Methodology: Literature data on the use of NSAIDs in domestic animals as chemo-preventive agents in the last decade were collected and critically reviewed. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Oxaliplatin, 5-Fluorouracil and Leucovorin (FOLFOX) As Second- Line Therapy for Patients with Advanced Urothelial Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 7, No. 36 Clinical Research Paper Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) as second- line therapy for patients with advanced urothelial cancer Sheng Zhang1, Hongxi Xue2, Qiang Chen3 1Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China 2Rizhao City Hospital of Traditional Chinese Medicine, Rizhao, China 3Department of Clinical Biochemistry, School of Public Health, Taishan Medical University, Tai’an, China Correspondence to: Sheng Zhang, email: [email protected] Keywords: urothelial cancer, oxaliplatin, leucovorin, 5-fluorouracil, clinical trial Received: February 08, 2016 Accepted: June 30, 2016 Published: July 07, 2016 ABSTRACT There is currently no standard treatment for metastatic urothelial cancer after failure of cisplatin-based therapy. The present retrospective study investigated the efficacy and safety of oxaliplatin plus 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX) in locally advanced or metastatic urothelial cancer patients following cisplatin-based treatment. Thirty-three patients who had received one or two cisplatin-based regimens were treated with oxaliplatin (85 mg/m2) as a 2-h infusion on day 1, LV (200 mg/m2) as a 2-h infusion followed by bolus 5-FU (400 mg/m2) on day 1, or a 44-h continuous 5-FU (1,200 mg/m2) infusion. Patients were a mean of 67 years old with two involved organs. Metastases were mostly in the lung (43%), lymph nodes (51%) and liver (46%). Based on an intention-to-treat analysis, nine patients achieved a partial response, with an overall response rate of 27%. Eight (24%) patients had stable disease. -

Eliminating Vincristine Administration Events
Issue 37 October 2017 Eliminating vincristine administration events Issue: Despite the usually deadly consequences of accidentally administering the chemotherapy drug vincristine intrathecally, adverse events still occur, typically because some organizations still administer vincristine via syringe. The good news is that these events are happening less in the United States, mostly because of the efforts of leading national organizations to promote an effective prevention strategy that assures a mechanical barrier to intrathecal administration (into the subarachnoid space). The strategy involves diluting intravenous vincristine or other vinca alkaloids in a minibag that contains a volume that is too large for intrathecal administration (e.g., 25 mL for pediatric patients and 50 mL for adults), making it mechanically difficult to accidentally administer intrathecally.1 Vinca alkaloids (vinblastine, vinorelbine, vincristine, and vincristine liposomal) are chemotherapy drugs that are intended to be administered intravenously. If given intrathecally, vincristine is nearly always fatal and associated with an irreversible, painful ascending paralysis.2 When vinca alkaloids are injected intrathecally, destruction of the central nervous system occurs, radiating out from the injection site. The few survivors of this adverse event experienced devastating neurological damage.1 Part of the problem stems from ordering intravenous vincristine in conjunction with medications that are administered intrathecally via a syringe, such as methotrexate, cytarabine and hydrocortisone. In some adverse events, vincristine was mistakenly injected into the cerebrospinal fluid (CSF) of patients when the intent was to inject another intrathecal chemotherapy agent, such as methotrexate or cytarabine.3 Intravenous vincristine and other vinca alkaloids are dispensed from the pharmacy with explicit warning labels about their lethality if given intrathecally.