VINBLASTINE-VINCRISTINE (Chlvpp-EVA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
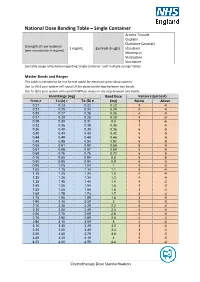
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

LEUKERAN 3 (Chlorambucil) 4 Tablets 5
1 PRESCRIBING INFORMATION ® 2 LEUKERAN 3 (chlorambucil) 4 Tablets 5 6 WARNING 7 LEUKERAN (chlorambucil) can severely suppress bone marrow function. Chlorambucil is a 8 carcinogen in humans. Chlorambucil is probably mutagenic and teratogenic in humans. 9 Chlorambucil produces human infertility (see WARNINGS and PRECAUTIONS). 10 DESCRIPTION 11 LEUKERAN (chlorambucil) was first synthesized by Everett et al. It is a bifunctional 12 alkylating agent of the nitrogen mustard type that has been found active against selected human 13 neoplastic diseases. Chlorambucil is known chemically as 4-[bis(2- 14 chlorethyl)amino]benzenebutanoic acid and has the following structural formula: 15 16 17 18 Chlorambucil hydrolyzes in water and has a pKa of 5.8. 19 LEUKERAN (chlorambucil) is available in tablet form for oral administration. Each 20 film-coated tablet contains 2 mg chlorambucil and the inactive ingredients colloidal silicon 21 dioxide, hypromellose, lactose (anhydrous), macrogol/PEG 400, microcrystalline cellulose, red 22 iron oxide, stearic acid, titanium dioxide, and yellow iron oxide. 23 CLINICAL PHARMACOLOGY 24 Chlorambucil is rapidly and completely absorbed from the gastrointestinal tract. After single 25 oral doses of 0.6 to 1.2 mg/kg, peak plasma chlorambucil levels (Cmax) are reached within 1 hour 26 and the terminal elimination half-life (t½) of the parent drug is estimated at 1.5 hours. 27 Chlorambucil undergoes rapid metabolism to phenylacetic acid mustard, the major metabolite, 28 and the combined chlorambucil and phenylacetic acid mustard urinary excretion is extremely 29 low — less than 1% in 24 hours. In a study of 12 patients given single oral doses of 0.2 mg/kg of 30 LEUKERAN, the mean dose (12 mg) adjusted (± SD) plasma chlorambucil Cmax was 31 492 ± 160 ng/mL, the AUC was 883 ± 329 ng●h/mL, t½ was 1.3 ± 0.5 hours, and the tmax was 32 0.83 ± 0.53 hours. -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

Chemotherapy in Advanced Ovarian Cancer: an Overview of Randomised Clinical Trials BMJ: First Published As 10.1136/Bmj.303.6807.884 on 12 October 1991
Chemotherapy in advanced ovarian cancer: an overview of randomised clinical trials BMJ: first published as 10.1136/bmj.303.6807.884 on 12 October 1991. Downloaded from Advanced Ovarian Cancer Trialists Group Abstract cytotoxic drugs, usually doxorubicin and cyclophos- Objectives-To consider the role of platinum and phamide with or without hexamethylmelamine.45 the relative merits of single agent and combination When, however, doubt was cast on the effectiveness chemotherapy in the treatment of advanced ovarian of doxorubicin by both randomised phase III trials6-8 cancer. and phase II studies in patients not responding to Design-Formal quantitative overview using cisplatin9 several major centres adopted cisplatin plus updated individual patient data from all available cyclophosphamide as standard. Furthermore, when randomised trials (published and unpublished). other trials failed to find significant survival differences Subjects-8139 patients (6408 deaths) included in between single agent cisplatin and cisplatin in combi- 45 different trials. nation with other drugs'0 some centres reverted to Results-No firm conclusions could be reached. using single agent platinum as routine first line treat- Nevertheless, the results suggest that in terms of ment. Recently a large number oftrials have compared survival immediate platinum based treatment was cisplatin with its less nephrotoxic and neurotoxic better than non-platinum regimens (overall relative analogue carboplatin, and although follow up times risk 0-93; 95% confidence interval 0-83 to 1-05); were often short, many institutions have now adopted platinum in combination was better than single agent carboplatin as standard. platinum when used in the same dose (overall Currently it is unclear what constitutes optimal relative risk 0-85; 0*72 to 1-00); and cisplatin and chemotherapy for advanced disease and treatment carboplatin were equally effective (overall relative strategies vary both nationally and internationally. -
B. Package Leaflet
B. PACKAGE LEAFLET 1 Package Leaflet: Information for the User Chlorambucil 2 mg tablets chlorambucil Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions ask your doctor or nurse. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Chlorambucil is and what it is used for 2. What you need to know before you take Chlorambucil 3. How to take Chlorambucil 4. Possible side effects 5. How to store Chlorambucil 6. Contents of the pack and other information 1. What Chlorambucil is and what it is used for Chlorambucil contains a medicine called chlorambucil. This belongs to a group of medicines called cytotoxics (also called chemotherapy). Chlorambucil is used to treat some types of cancer and certain blood problems. It works by reducing the number of abnormal cells your body makes. Chlorambucil is used for: - Hodgkin's disease and Non-Hodgkin’s Lymphoma. Together, these form a group of diseases called lymphomas. They are cancers formed from cells of the lymphatic system. - Chronic lymphocytic leukaemia. A type of white blood cell cancer where the bone marrow produces a large number of abnormal white cells. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Eliminating Vincristine Administration Events
Issue 37 October 2017 Eliminating vincristine administration events Issue: Despite the usually deadly consequences of accidentally administering the chemotherapy drug vincristine intrathecally, adverse events still occur, typically because some organizations still administer vincristine via syringe. The good news is that these events are happening less in the United States, mostly because of the efforts of leading national organizations to promote an effective prevention strategy that assures a mechanical barrier to intrathecal administration (into the subarachnoid space). The strategy involves diluting intravenous vincristine or other vinca alkaloids in a minibag that contains a volume that is too large for intrathecal administration (e.g., 25 mL for pediatric patients and 50 mL for adults), making it mechanically difficult to accidentally administer intrathecally.1 Vinca alkaloids (vinblastine, vinorelbine, vincristine, and vincristine liposomal) are chemotherapy drugs that are intended to be administered intravenously. If given intrathecally, vincristine is nearly always fatal and associated with an irreversible, painful ascending paralysis.2 When vinca alkaloids are injected intrathecally, destruction of the central nervous system occurs, radiating out from the injection site. The few survivors of this adverse event experienced devastating neurological damage.1 Part of the problem stems from ordering intravenous vincristine in conjunction with medications that are administered intrathecally via a syringe, such as methotrexate, cytarabine and hydrocortisone. In some adverse events, vincristine was mistakenly injected into the cerebrospinal fluid (CSF) of patients when the intent was to inject another intrathecal chemotherapy agent, such as methotrexate or cytarabine.3 Intravenous vincristine and other vinca alkaloids are dispensed from the pharmacy with explicit warning labels about their lethality if given intrathecally. -
Efficacy of Ethinylestradiol Re-Challenge for Metastatic Castration-Resistant Prostate Cancer
ANTICANCER RESEARCH 36: 2999-3004 (2016) Efficacy of Ethinylestradiol Re-challenge for Metastatic Castration-resistant Prostate Cancer TAKEHISA ONISHI1, TAKUJI SHIBAHARA1, SATORU MASUI1, YUSUKE SUGINO1, SHINICHIRO HIGASHI1 and TAKESHI SASAKI2 1Department of Urology, Ise Red Cross hospital, Ise, Japan; 2Department of Urology, Mie University Graduate School of Medicine, Tsu, Japan Abstract. Background: There has recently been renewed corticosteroids, estrogens, sipuleucel T and, more recently, interest in the use of estrogens as a treatment strategy for CYP17 inhibitor (abirateron acetate) and androgen castration-resistant prostate cancer (CRPC). The purpose of receptor antagonist (enzaltamide) (2-7). Treatment with this study was to evaluate the feasibility and efficacy of estrogens was used as a palliative therapy for advanced ethinylestradiol re-challenge (re-EE) in the management of prostate cancer, however, the discovery of luteinizing CRPC. Patients and Methods: Patients with metastatic CRPC hormone-releasing hormone (LH-RH) agonists led them to who received re-EE after disease progression on prior EE become less common and they stopped being used in most and other therapy were retrospectively reviewed for prostate- countries in the 1980s (8). One of the reasons for reduction specific antigen (PSA) response, PSA progression-free in their use is the risk of cardiovascular and survival (P-PFS) and adverse events. Results: Thirty-six re- thromboembolic events during therapy. However, several EE treatments were performed for 20 patients. PSA response reports demonstrated the positive oncological results of to the initial EE treatment was observed in 14 (70%) patients. therapy with estrogens, such as diethylstilbestrol (DES) PSA response to re-EE was 33.3% in 36 re-EE treatments. -

Hematologic Oncology Update — Issue 1, 2014
POST-TEST Hematologic Oncology Update — issue 1, 2014 The c o rrec T answer is indicaTed wiTh yellow highlighTin g. 1. Which of the following is true regarding the 6. A Phase II study of single-agent brentux- CRS related to CAR T-cell therapy? imab vedotin for the treatment of relapsed a. It manifests as fever, nausea, myalgia or refractory CD30-positive non-hodgkin and hypotension lymphomas demonstrated promising antitumor b. It is associated with high levels of IL-6 activity in patients with DLBCL with a broad c. It is irreversible range of CD30 expression levels. d. Both a and b a. True b. False 2. Updated results of a pilot trial by Porter and colleagues of chimeric antigen receptor T-cell 7. Results from a Phase II trial comparing therapy for patients with relapsed/refractory consolidation therapy with a single dose of CLL reported an overall response rate of 90y-ibritumomab tiuxetan to rituximab mainte- _____________. nance for patients with newly diagnosed Fl a. 12% who have experienced a response to r-CHOP b. 57% demonstrated statistically significant differ- ences in _____________ favoring rituximab c. 82% maintenance. 3. Tocilizumab, an IL-6 receptor antagonist, is a. Progression-free survival effective at reversing the cytokine release b. Overall survival syndrome associated with chimeric antigen c. Both a and b receptor-modified T-cell therapy. a. True 8. The final stage II results of the Phase b. False III CLL11 trial for patients with CLL and coexisting medical conditions demon- 4. The results of a Phase III study of induction strated that obinutuzumab/chlorambucil was therapy with lenalidomide and dexametha- superior to rituximab/chlorambucil in terms of sone followed by maintenance lenalidomide _____________. -

Essential Thrombocythemia Facts No
Essential Thrombocythemia Facts No. 12 in a series providing the latest information for patients, caregivers and healthcare professionals www.LLS.org • Information Specialist: 800.955.4572 Introduction Highlights Essential thrombocythemia (ET) is one of several l Essential thrombocythemia (ET) is one of a related “myeloproliferative neoplasms” (MPNs), a group of closely group of blood cancers known as “myeloproliferative related blood cancers that share several features, notably the neoplasms” (MPNs) in which cells in the bone “clonal” overproduction of one or more blood cell lines. marrow that produce the blood cells develop and All clonal disorders begin with one or more changes function abnormally. (mutations) to the DNA in a single cell; the altered cells in l ET begins with one or more acquired changes the marrow and the blood are the offspring of that one (mutations) to the DNA of a single blood-forming mutant cell. Other MPNs include polycythemia vera and cell. This results in the overproduction of blood cells, myelofibrosis. especially platelets, in the bone marrow. The effects of ET result from uncontrolled blood cell l About half of individuals with ET have a mutation production, notably of platelets. Because the disease arises of the JAK2 (Janus kinase 2) gene. The role that this from a change to an early blood-forming cell that has the mutation plays in the development of the disease, capacity to form red cells, white cells and platelets, any and the potential implications for new treatments, combination of these three cell lines may be affected – and are being investigated. usually each cell line is affected to some degree.