Preferred Drug List 4-Tier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

XTANDI® (Enzalutamide): a Treatment Option for Castration-Resistant Prostate Cancer (CRPC)
XTANDI® (enzalutamide): A Treatment Option for Castration-Resistant Prostate Cancer (CRPC) XTANDI® (enzalutamide) is approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with castration-resistant prostate cancer (CRPC).1 XTANDI is the first and only oral medication FDA-approved for both non-metastatic and metastatic CRPC.1 ABOUT THE ASTELLAS/PFIZER HOW XTANDI WORKS COLLABORATION Astellas and Pfizer jointly XTANDI is indicated for the treatment of CRPC, commercialize XTANDI which is defined as disease progression on in the United States. androgen deprivation therapy (luteinizing hormone-releasing hormone (LHRH) therapy Astellas has responsibility or prior bilateral orchiectomy).2 for manufacturing and all additional regulatory In prostate cancer, the androgen receptor (AR) is a key driver filings globally, as well as of progression.3 XTANDI is an AR inhibitor that is thought commercializing XTANDI to act on multiple steps of the androgen receptor signaling 1 outside the United States. pathway within the tumor cell based on in vitro studies. Select Safety Information Seizure occurred in 0.4% of patients receiving XTANDI in clinical studies. In a study of patients with predisposing factors for seizure, 2.2% of XTANDI-treated patients experienced a seizure. Patients in the study had one or more of the following pre-disposing factors: use of medications that may lower the seizure threshold; history of traumatic brain or head injury, cerebrovascular accident or transient ischemic attack, Alzheimer’s disease, meningioma, or leptomeningeal disease from prostate cancer, unexplained loss of consciousness within the last 12 months, history of seizure, presence of a space occupying lesion of the brain, history of arteriovenous malformation, or history of brain infection. -

Flexible Plasmonic Sensing Substrates and Their Application in Explosive Sensing Justin Bae Washington University in St Louis
Washington University in St. Louis Washington University Open Scholarship Engineering and Applied Science Theses & McKelvey School of Engineering Dissertations Spring 5-17-2017 Flexible Plasmonic Sensing Substrates and their application in Explosive Sensing Justin Bae Washington University in St Louis Srikanth Singamaneni Washington University in Saint Louis Follow this and additional works at: https://openscholarship.wustl.edu/eng_etds Part of the Engineering Commons Recommended Citation Bae, Justin and Singamaneni, Srikanth, "Flexible Plasmonic Sensing Substrates and their application in Explosive Sensing" (2017). Engineering and Applied Science Theses & Dissertations. 231. https://openscholarship.wustl.edu/eng_etds/231 This Thesis is brought to you for free and open access by the McKelvey School of Engineering at Washington University Open Scholarship. It has been accepted for inclusion in Engineering and Applied Science Theses & Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS School of Engineering and Applied Science Department of Mechanical Engineering and Materials Science Thesis Examination Committee: Srikanth Singamaneni, Chair Guy Genin Jeremiah Morrissey Flexible Plasmonic Sensing Substrates and their application in Explosive Sensing by Sang hyun Justin Bae A thesis presented to the School of Engineering of Washington University in St. Louis in partial fulfillment of the requirements for the -

Gastrointestinal (GI) Motility, Chronic Therapeutic Class Review
Gastrointestinal (GI) Motility, Chronic Therapeutic Class Review (TCR) March 7, 2019 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. March 2019 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004–2019 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) alosetron (Lotronex®)1 generic, . -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log
Clinical Policy: Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Lindane is an ectoparasiticide and ovicide effective against Pediculus humanus capitis (head lice), Pthirus pubis (crab lice), and their ova. FDA Approved Indication(s) Lindane Shampoo is indicated for the treatment of head lice (infestations of Pediculus humanus capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Lindane shampoo is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Head Lice (must meet all): 1. Diagnosis of Pediculus capitis (head lice); 2. Failure of two preferred agents indicated for head lice (see Appendix B for examples), at least one of which was used within the past 60 days, unless ALL preferred agents for head lice are contraindicated or clinically significant adverse effects are experienced; 3. Request does not exceed 1 bottle (60 mL) per treatment course. Approval duration: 14 days B. Crab Lice (must meet all): 1. Diagnosis of Phthirus pubis (crab lice); 2. Failure of pyrethrins/piperonyl butoxide AND permethrin 1% cream, used in last 60 days, unless both are contraindicated or clinically significant adverse effects are experienced; 3. -

Lindane Lotion USP, 1% RX Only WARNINGS
Lindane Lotion USP, 1% RX Only WARNINGS: Lindane Lotion should only be used in patients who cannot tolerate or have failed first-line treatment with safer medications for the treatment of scabies. (See INDICATIONS AND USAGE.) Neurologic Toxicity Seizures and deaths have been reported following Lindane Lotion use with repeat or prolonged application, but also in rare cases following a single application used according to directions. Lindane lotion should be used with caution for infants, children, the elderly, and individuals with other skin conditions (e.g, atopic dermatitis, psoriasis) and in those who weigh < 110 lbs (50 kg) as they may be at risk of serious neurotoxicity. Contraindications Lindane Lotion is contraindicated in premature infants and individuals with known uncontrolled seizure disorders. Proper Use Instruct patients on the proper use of Lindane Lotion, the amount to apply, how long to leave it on, and avoiding re-treatment. Inform patients that itching occurs after the successful killing of scabies and is not necessarily an indication for re-treatment with Lindane Lotion. (See DOSAGE AND ADMINISTRATION.) DESCRIPTION Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide. In addition to the active ingredient, lindane, it contains glycerol monostearate, cetyl alcohol, stearic acid, trolamine, carrageenan, 2- amino-2-methyl-1-propanol, methylparaben, butylparaben, perfume and water to form a lotion. Lindane is the gamma isomer of 1,2,3,4,5,6-hexachlorocyclohexane having the following structural formula: C6H6Cl6 M.W. 290.83 CLINICAL PHARMACOLOGY Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide effective against Sarcoptes scabiei (scabies). Lindane exerts its parasiticidal action by being directly absorbed into the parasites and 1 their ova. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Clinical Efficacy of Enzalutamide Vs Bicalutamide Combined with Androgen Deprivation Therapy in Men with Metastatic Hormone-Sens
Henry Ford Health System Henry Ford Health System Scholarly Commons Hematology Oncology Articles Hematology-Oncology 1-4-2021 Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone- Sensitive Prostate Cancer: A Randomized Clinical Trial Ulka N. Vaishampayan Lance K. Heilbrun Paul Monk Sheela Tejwani Guru Sonpavde See next page for additional authors Follow this and additional works at: https://scholarlycommons.henryford.com/ hematologyoncology_articles Authors Ulka N. Vaishampayan, Lance K. Heilbrun, Paul Monk, Sheela Tejwani, Guru Sonpavde, Clara Hwang, Daryn Smith, Pallavi Jasti, Kimberlee Dobson, Brenda Dickow, Elisabeth I. Heath, Louie Semaan, Michael L. Cher, Joseph A. Fontana, and Sreenivasa Chinni Original Investigation | Oncology Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone-Sensitive Prostate Cancer A Randomized Clinical Trial Ulka N. Vaishampayan, MD; Lance K. Heilbrun, PhD; Paul Monk III, MD; Sheela Tejwani, MD; Guru Sonpavde, MD; Clara Hwang, MD; Daryn Smith, MS; Pallavi Jasti, MD; Kimberlee Dobson, BS; Brenda Dickow, RN; Elisabeth I. Heath, MD; Louie Semaan, BS; Michael L. Cher, MD; Joseph A. Fontana, MD; Sreenivasa Chinni, PhD Abstract Key Points Question Is enzalutamide combined IMPORTANCE Black patients have been underrepresented in prospective clinical trials of advanced with androgen deprivation therapy prostate cancer. This study evaluated the efficacy of enzalutamide compared with bicalutamide, with associated with better outcomes than planned subset analysis of Black patients with metastatic hormone-sensitive prostate cancer treatment with bicalutamide in Black (mHSPC), which is a disease state responsive to androgen deprivation therapy (ADT). men with metastatic hormone-sensitive prostate cancer (mHSPC)? OBJECTIVE To compare the efficacy of enzalutamide vs bicalutamide in combination with ADT in men with mHSPC, with a subset analysis of Black patients. -
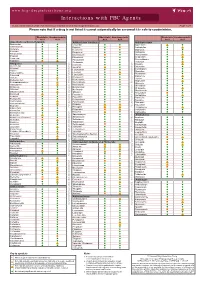
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

Vaginal Delivery System
(19) & (11) EP 2 062 568 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 27.05.2009 Bulletin 2009/22 A61K 9/00 (2006.01) (21) Application number: 07397042.8 (22) Date of filing: 22.11.2007 (84) Designated Contracting States: • Hanes, Vladimir AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Tarrytown, NY 10591 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE • Keinänen, Antti SI SK TR 20540 Turku (FI) Designated Extension States: • Holmberg, Svante AL BA HR MK RS 20900 Turku (FI) • Nikander, Hannu (71) Applicant: Bayer Schering Pharma Oy 21330 Paattinen (FI) 20210 Turku (FI) (74) Representative: Matilainen, Mirja Helena et al (72) Inventors: Oy Jalo Ant-Wuorinen AB, • Talling, Christine Iso Roobertinkatu 4-6 A 20610 Turku (FI) 00120 Helsinki (FI) (54) Vaginal delivery system (57) The present invention is related to an intravag- brane (3) encasing the core, said core and membrane inal delivery system for the controlled release of a pro- essentially consisting of a same or different polymer com- gestogen and an estrogen, comprising additionally a position, wherein at cast one of the cores comprises a therapeutically active or a health-promoting substance progestogen or a mixture of a progestogen and an es- (1) capable of giving and/or enhancing the protection trogen, and another core may comprise an estrogen or against bacterial and fungal infections, and/or enhancing a progestogen, and wherein the membrane or the surface the protection against sexually transmitted diseases. The of the membrane or at least one of the cores comprises delivery system consists of one or more compartments said therapeutically active or a health-promoting sub- (2,4,5), one of each comprising a core (7) and a mem- stance. -

Stiripentol with an Average Age of 9.1 Years
VOLUME 43 : NUMBER 3 : JUNE 2020 NEW DRUGS The other trial was in Italy and involved 23 children Stiripentol with an average age of 9.1 years. After two months, eight children responded to stiripentol (66.7%). Aust Prescr 2020;43:102 Approved indication: Dravet syndrome Only one (9.1%) in the placebo group responded. https://doi.org/10.18773/ Diacomit (Emerge Health) Three of the children taking stiripentol became free austprescr.2020.029 250 mg and 500 mg capsules of seizures.2 First published 250 mg and 500 mg powder for oral suspension During the trials there were more adverse events in 24 April 2020 Dravet syndrome is a severe myoclonic epilepsy in the children taking stiripentol, compared to placebo. infancy. It usually emerges in the first year of life. More frequent effects included drowsiness, agitation, The seizures are difficult to control and the infants irritability, hypotonia, nausea and vomiting. There develop intellectual disability. Stiripentol has been was also loss of appetite and weight loss. Elevation approved as an adjunctive treatment for infants with of gamma-glutamyltransferase has been reported so generalised tonic-clonic and clonic seizures which are liver function should be checked every six months, not controlled by valproate and a benzodiazepine. as should a full blood count because of the risk of neutropenia. Stiripentol is an aromatic alcohol which is unrelated to the structure of other antiepileptic drugs. It While it is difficult to know if the effect is due to its increases activity in the GABAergic system, but it also interaction with clobazam and valproate, stiripentol interacts with anticonvulsant drugs.