Lindane Lotion USP, 1% RX Only WARNINGS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log
Clinical Policy: Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Lindane is an ectoparasiticide and ovicide effective against Pediculus humanus capitis (head lice), Pthirus pubis (crab lice), and their ova. FDA Approved Indication(s) Lindane Shampoo is indicated for the treatment of head lice (infestations of Pediculus humanus capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Lindane shampoo is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Head Lice (must meet all): 1. Diagnosis of Pediculus capitis (head lice); 2. Failure of two preferred agents indicated for head lice (see Appendix B for examples), at least one of which was used within the past 60 days, unless ALL preferred agents for head lice are contraindicated or clinically significant adverse effects are experienced; 3. Request does not exceed 1 bottle (60 mL) per treatment course. Approval duration: 14 days B. Crab Lice (must meet all): 1. Diagnosis of Phthirus pubis (crab lice); 2. Failure of pyrethrins/piperonyl butoxide AND permethrin 1% cream, used in last 60 days, unless both are contraindicated or clinically significant adverse effects are experienced; 3. -

Revised Use-Function Classification (2007)
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY IPCS INTOX Data Management System (INTOX DMS) Revised Use-Function Classification (2007) The Use-Function Classification is used in two places in the INTOX Data Management System: the Communication Record and the Agent/Product Record. The two records are linked: if there is an agent record for a Centre Agent that is the subject of a call, the appropriate Intended Use-Function can be selected automatically in the Communication Record. The Use-Function Classification is used when generating reports, both standard and customized, and for searching the case and agent databases. In particular, INTOX standard reports use the top level headings of the Intended Use-Functions that were selected for Centre Agents in the Communication Record (e.g. if an agent was classified as an Analgesic for Human Use in the Communication Record, it would be logged as a Pharmaceutical for Human Use in the report). The Use-Function classification is very important for ensuring harmonized data collection. In version 4.4 of the software, 5 new additions were made to the top levels of the classification provided with the system for the classification of organisms (items XIV to XVIII). This is a 'convenience' classification to facilitate searching of the Communications database. A taxonomic classification for organisms is provided within the INTOX DMS Agent Explorer. In May/June 2006 INTOX users were surveyed to find out whether they had made any changes to the Use-Function Classification. These changes were then discussed at the 4th and 5th Meetings of INTOX Users. Version 4.5 of the INTOX DMS includes the revised pesticides classification (shown in full below). -

FLORA-FOG MALATHION-LINDANE~-A~CCEP-TF.:W: SEE LEFT PANEL FOR~~:!~~: and PRECAUTIONS ~
• , DANGER! KEEP OUT OF I~EACH OF CHILDREN! . e·. ... '---. FLORA-FOG MALATHION-LINDANE~-A~CCEP-TF.:w: SEE LEFT PANEL FOR~~:!~~: AND PRECAUTIONS ~ . -' . PRECAUTIONS b-'7---~¥ £2rl-l~ : r 1IT. t • . 00 ANTIDOTES: £12 AL INS£CflClOE . " .' ~' ...... -.. mm~ TWo F~D' ':0' DEN'flCIDE ACI . I If swallowed: Give a tablespoonful.of salt 111 a glass of warm water an ...... ,..,.... X'" • t, " F'';~' :.;,~ ":""'~'C pOISON R&G1S~!a\' repeat until vomit flUid IS clear Have vlCtml lie down and keep qUiet FO" •.. '. .. Call a physIcian immediately I ED ...~jNt-i. HO~• ....1~~~---- If on skin: Wash immediately with soap and water GREENHOUSE FOGG.lNG INSECTICIDE Atropine is antidotal FORMULATED SPECIFICALLY FOR liSE IN THERMAL FOG GENERATORS HIGHLY CONCENTRATED INSECTICIDE FOR THE CONTROL OF INSECTS AND MITES DESTRUCTIVE TO GREENHOUSE ORNAMENTALS, SUCH AS CARNATIONS, CHRYSAriTHEMUMS, ROSES, SNAPDRAGONS, AND GERANIUMS WARNING: NON·INJURIOUS TO EITHER BLOSSOMS OR I-OLIAGE WHEN USED AS DIRECTED • Poisonous by skill contact. If)i1alatlon. or sWillloWlnql RClpidly .:Jbsorued Will DESTRlY through Sklnl Do not get If) eyes. Oil skll1 or on clotlHn~1 In case of Red Spider 1'v1ltes. certain ether !]lItes. Aphids. Le.lfilOfwers. Thrips. Ntlltetiles. I'vkdlyhlJf:5. CucullltJer 8eet:es. ChrysilntllelllUiI1 contact, immediately remove illl contamtnatf'd clothlnq and flush sktn l ('itt 1\ll1il'r~. Orchid Wee,;iI. Plt),l1€' Mt ths. and Soft Sc.lle Inst~ct5 or eyes with plenty of water for at le.lst 15 minutes For eyes. get ACTIVE INGREDIENTS 100": , medical attention I Do not breathe fO~1 W d~h thorouqhly with SOilP and Malathion ........................................................................................... -

Antibiotics May Eradicate Gastrointestinal Immunodeficiency.8
1122 Letters to the Editor J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.54.12.1122 on 1 December 1991. Downloaded from sitivity.? We present a case of OMM without pseudobulbar palsy. The association of vitro.8 Whipple's disease is associated with symptoms of Whipple's disease and with supranuclear gaze paresis with oculomas- immunodeficiency.8 Thus intestinal wall negative peroral jejunoileal biopsies, which ticatory myorhythmia, however, seems to be macrophages are ineffective in phagocytosing indicates the usefulness of laparotomy for pathognomonic of the effects of Whipple's intracellular gram positive bacilli, resulting in jejunoileal biopsies as an alternative to brain disease on the CNS.'2 This led us to perform inability to eliminate chronic infection.9 This biopsy to confirm Whipple's disease. surgical jejunal and mesenteric lymph node suggests that Whipple's disease may be con- A 47 year old woman was admitted in May biopsies rather than a brain biopsy despite sidered as a disease of macrophages.8 The 1988 in a depressed state. In June 1987 she negative endoscopic and numerous peroral periventricular and periaqueductal distribu- had noted progressive visual disturbance. distal jejunal and ileal biopsies. The nor- tion of the CNS involvement in Whipple's Rhythmic elevations of her right upper lip mality ofthe CT and MRI scans also suppor- disease consists of macrophagic infiltration appeared, and later, paroxysmal hypersomnia ted this decision. and subependymal nodules. Such a and considerable weight gain (10 kg). A nine The possibility of brain involvement with- "tumoural" involvement may explain why month course oftreatment for depression was out systemic manifestation in Whipple's dis- antibiotics with good BBB diffusion are not ineffective and her symptoms and signs ease should be kept in mind. -
PASS Information
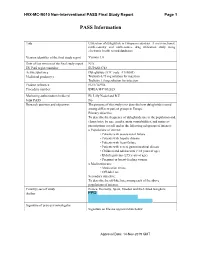
H9X-MC-B010 Non-interventional PASS Final Study Report Page 1 PASS Information Title Utilisation of dulaglutide in European countries: A cross-sectional, multi-country and multi-source drug utilisation study using electronic health record databases Version identifier of the final study report Version 1.0 Date of last version of the final study report N/A EU PAS register number EUPAS13783 Active substance Dulaglutide (ATC code: A10BJ05) Medicinal product(s): Trulicity 0.75 mg solution for injection Trulicity 1.5 mg solution for injection Product reference: EU/1/14/956 Procedure number: EMEA/H/C/002825 Marketing authorisation holder(s) Eli Lilly Nederland B.V. Joint PASS No Research question and objectives The purpose of this study is to describe how dulaglutide is used among different patient groups in Europe. Primary objective: To describe the frequency of dulaglutide use in the population and characterise by age, gender, main comorbidities, and main co- prescriptions overall and in the following subgroups of interest: o Populations of interest: • Patients with severe renal failure • Patients with hepatic disease • Patients with heart failure • Patients with severe gastrointestinal disease • Children and adolescents (<18 years of age) • Elderly patients (≥75 years of age) • Pregnant or breast-feeding women o Medication use: • Medication errors • Off-label use Secondary objective: To describe the off-label use among each of the above populations of interest. Country(-ies) of study France, Germany, Spain, Sweden and the United Kingdom Author PPD Signature of principal investigator Signature on file/see approval date below Approval Date: 14-Nov-2019 GMT H9X-MC-B010 Non-interventional PASS Final Study Report Page 2 Marketing Authorisation Holder Marketing authorisation holder (MAH) Eli Lilly Nederland B.V. -

A Saprolegnia Parasitica Challenge System for Rainbow Trout: Assessment of Pyceze As an Anti-Fungal Agent for Both Fish and Ova
DISEASES OF AQUATIC ORGANISMS Published May 12 Dis Aquat Org l A Saprolegnia parasitica challenge system for rainbow trout: assessment of Pyceze as an anti-fungal agent for both fish and ova T. G.Pottinger*, J. G. Day NERC Institute of Freshwater Ecology, Windermere Laboratory, Far Sawrey, Ambleside, Cumbria, LA22 OLP, United Kingdom ABSTRACT: A reproducible Saprolegnia parasitica spore delivery system was developed and demon- strated to be effective in providing a sustained spore challenge for up to 10 d. Treatment of rainbow trout with slow-release intraperitoneal implants containing cortisol resulted in chronically elevated blood cortisol levels and rendered the fish susceptible to infection by S. parasitica when exposed to the spore challenge. Sham-implanted fish were not susceptible to infect~on.Bronopol (2-bromo-2-nitro- propane-1,3-dol), formulated as Pyceze, was effective in protecting predisposed fish from infection by S. parasitica when administered as a daily bathlflush treatment at concentrations of 15 mg I-' and greater. Pyceze was also demonstrated to protect fertilised rainbow trout ova from S. parasitica chal- lenge when administered as a daily bath/flush treatment at concentrations of between 30 and 100 mg 1-l. Pyceze appears to qualify as a safe and effective replacement for malachite green and formalin in the prevention of fungal infections in the aquaculture environment. KEY WORDS: Saprolegnia . Fungal infection . Salmonid . Bronopol - Pyceze . Cortisol INTRODUCTION chite green in combating mycotic infections of fish and fish eggs, but is safer for the operator, the fish, and Mycotic infections of farmed fish, primarily by water the environment. Among the alternative compounds moulds or pseudofungi of the genus Saprolegnia, rep- tested for antifungal activity are sodium chloride resent a significant economic and welfare problem. -

Wood-Boring Insects of Trees and Shrubs
B- 508 6 Wood-boring Insects of Trees and Shrubs Bastiaan M. Drees, Professor and Extension Entomologist John A. Jackman, Professor and Extension Entomologist Michael E. Merchant, Assistant Professor and Extension Urban Entomologist The Texas A&M University System Many insects feed and make their homes in the bark, trunks and branches of shade trees and shrubs in Texas. Bark beetles and insect borers belong to several different insect groups including a variety of beetles, moths and horntail wasps. Most insect borers are attracted to weakened, damaged, dying or dead plants. These are referred to as “secondary invaders” because they attack only after a plant has been weakened by another stress. Secondary invaders are a symptom of other problems with the health of the tree or shrub, but may contribute to its decline. Secondary invaders include species from groups already mentioned, but also may include termites, carpenter bees and carpenter ants. Many other insects live in dying or dead trees, including natural enemies (predators and parasites) of the insect borers, sap or fungi feeders, or species which merely use the spaces provided by the tunnels and galleries as living quarters. Wood-boring insects that attack healthy trees and shrubs are called “primary invaders.” Primary invaders may eventually kill trees. Damage Borer infestations often go unnoticed until plants or parts of plants begin to die or show external signs of damage. Wood-boring insects often produce sawdust-like frass (excrement). Their holes are normally round, oval or semicircular and are found in a random pattern on the plant. Woodpecker damage is sometimes confused with that of wood-boring beetles, however woodpecker damage will not produce frass. -

A Single Amino Acid Substitution in the Third Transmembrane Region Has Opposite Impacts on the Selectivity of the Parasiticides
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/09/08/mol.117.109413.DC1 1521-0111/92/5/546–555$25.00 https://doi.org/10.1124/mol.117.109413 MOLECULAR PHARMACOLOGY Mol Pharmacol 92:546–555, November 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics A Single Amino Acid Substitution in the Third Transmembrane Region Has Opposite Impacts on the Selectivity of the Parasiticides Fluralaner and Ivermectin for Ligand-Gated Chloride Channels s Yunosuke Nakata, Toshinori Fuse, Kohei Yamato, Miho Asahi, Kunimitsu Nakahira, Fumiyo Ozoe, and Yoshihisa Ozoe Faculty of Life and Environmental Science, Shimane University, Matsue, Shimane, Japan (Y.N., T.F., K.Y, F.O., Y.O.); and Downloaded from Biological Research Laboratories, Nissan Chemical Industries, Ltd., Saitama, Japan (M.A., K.N.) Received May 16, 2017; accepted September 9, 2017 ABSTRACT Fluralaner (Bravecto) is a recently marketed isoxazoline ectopar- transmembrane region (TM3) with an aromatic amino acid molpharm.aspetjournals.org asiticide. This compound potently inhibits GABA-gated chloride dramatically enhanced the potency of fluralaner in the GluCls. channels (GABACls) and less potently glutamate-gated chloride In stark contrast to the enhancement of fluralaner potency, this channels (GluCls) in insects. The mechanism underlying this mutation eliminated the activation of currents and the potenti- selectivity is unknown. Therefore, we sought to identify the ation but not the antagonism of glutamate responses that are amino acid residues causing the low potency of fluralaner toward otherwise all elicited by the macrolide parasiticide ivermectin GluCls. We examined the fluralaner sensitivity of mutant housefly (IVM). -

Study Protocol
STUDY PROTOCOL FOR COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR 35% PEROX-AID® (HYDROGEN PEROXIDE) FOR THE CONTROL OF ECTOPARASITES (INAD #11-669) Sponsor: U.S. Fish and Wildlife Service, Division of the National Fish Hatchery System ______________________ ___________________ Sponsor Signature Date Approved Manufacturer: Eka Chemicals Inc. 1775 West Oak Commons Court Marietta, Georgia 30062-2254 Facility for Coordination of 35% PEROX AID® INAD: Aquatic Animal Drug Approval Partnership Program 4050 Bridger Canyon Road Bozeman, Mt 59715 Proposed Starting Date: December 1, 2007 Proposed Ending Date: November 30, 2012 Study Director: Mr. Jim Bowker _________________________ ________________ Study Director Signature Date Clinical Field Trial Location and Trial Number: Facility ______________________________________________________________ Type or Print Name Investigator____________________________________________________________ Type or Print Name __________________________________________ ________________________ Investigator Signature Date 1 I. STUDY ID AND TITLE 3 II. SPONSOR 3 III. INVESTIGATORS/FACILITIES 4 IV. PROPOSED STARTING AND COMPLETION DATES: 4 V. BACKGROUND/PURPOSE 4 VI. SPECIFIC OBJECTIVES 6 VII. MATERIALS 7 VIII. EXPERIMENTAL UNIT 10 IX. ENTRANCE CRITERIA 10 X. TREATMENT GROUPS 11 XI. TREATMENT SCHEDULES 12 XII. TREATMENT RESPONSE PARAMETERS 15 XIII. FORMS FOR DATA COLLECTION 15 XIV. RECORD KEEPING PROCEDURES 16 XV. DISPOSITION OF INVESTIGATIONAL ANIMALS 16 XVI. DISPOSITION OF INVESTIGATIONAL -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Laboratory and Field Studies to Investigate the Efficacy of a Novel
Kryda et al. Parasites Vectors (2019) 12:445 https://doi.org/10.1186/s13071-019-3702-6 Parasites & Vectors RESEARCH Open Access Laboratory and feld studies to investigate the efcacy of a novel, orally administered combination product containing moxidectin, sarolaner and pyrantel for the prevention of heartworm disease (Diroflaria immitis) in dogs Kristina Kryda1*, Robert H. Six1, Kelly F. Walsh1, Susan J. Holzmer1, Sara Chapin1, Sean P. Mahabir1, Melanie Myers1, Tammy Inskeep1, Jady Rugg1, Blair Cundif1, Aleah Pullins1, Michael Ulrich2, John W. McCall3, Tom L. McTier1 and Steven J. Maeder1 Abstract Background: Diroflaria immitis is a flarial parasite of dogs that can cause serious or fatal cardiopulmonary disease. Three studies were conducted to evaluate the efcacy and safety of monthly treatment with moxidectin in a chew- able tablet product in combination with sarolaner and pyrantel to prevent heartworm disease in dogs after experi- mental challenge and in a clinical feld study in the USA. Methods: In two laboratory studies, dogs (8 per group) that had been inoculated 30 days prior with 50 third-stage D. immitis larvae were randomized to treatment on Day 0 with placebo or combination product, at the minimum dose of 24 µg/kg moxidectin, 2 mg/kg sarolaner and 5 mg/kg pyrantel (as pamoate salt). Study 2 also included groups treated with tablets containing moxidectin-alone (24 µg/kg) or sarolaner-alone (2 mg/kg). Efcacy was evaluated ~ 5 months after inoculation by adult heartworm counts at necropsy. In the feld study, 410 dogs 8 weeks-old from 23 USA veterinary clinics were treated for 11 months with either combination product at 24–48 µg/kg≥ moxidectin, 2–4 mg/kg sarolaner and 5–10 mg/kg pyrantel (n 272) or Heartgard® Plus (ivermectin/pyrantel) at the label recom- mended dose rate (n 138).