Vaginal Delivery System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emerging Threat in Antifungal Resistance on Superficial Dermatophyte Infection R Sultana1, M Wahiduzzaman2
Bang Med J Khulna 2018; 51 : 21-24 ORIGINAL ARTICLE Emerging threat in antifungal resistance on superficial dermatophyte infection R Sultana1, M Wahiduzzaman2 Abstract Background: Dermatophytosis are most common fungul infection globally and according to WHO the prevalence is about 20-25% and does not spare people of any race or age. Over the past few years antifungal resistance has been emerged due to irrational use of antifungal drugs in cutaneous mycosis. Objective: The aim of the study is to evaluate the efficacy of different antifungul drugs (Terbinafin. Fluconazole, Itraconazole, Griseofulvin) on superficial mycosis depending on various factors. Methods: This prospective study was conducted among the Superficial fungul infected patients from April' 2017 to October 2017 in Khulna Medical College Hospital (KMCH) and dermatologist's private chamber in Khulna city. All the enrolled patients were put on oral Terbinafin, Fluconazole, Itraconazole and Griseofulvin. Each patient was given single antifungal drug orally. These cases were thus followed up after two months of treatment to look for persistent infection, cure or any relapse clinically. Result: Among 194 patient 89 were given Tab. Terbinafin (250mg) where resistance cases were 20.22%. More cases (33.96%) were resistant to Cap. Fluconazol (50mg). High percentage of cases were resistant to Cap. Itraconazole (76.47%). Griseofulvin resistant cases were observed in 25.71%. Drug response is very poor (69%) in patient who had been suffering from diabetes mellitus. Conclusion: Appropriate antifungal drugs should be chosen with strict indication, dose, duration, selection of perfect local preparation and taking laboratory facilities where necessary. Keywords : Dermatophytosis, Antifungal resistance, Public health. -

Substrate Inhibition of 17 Beta-Hydroxysteroid Dehydrogenase Type 1 in Living Cells and Regulation Among the Steroid-Converting Enzymes in Breast Cancers
Substrate inhibition of 17 beta-hydroxysteroid dehydrogenase type 1 in living cells and regulation among the steroid-converting enzymes in breast cancers Thèse Hui Han Doctorat en médecine moléculaire Philosophiæ doctor (Ph. D.) Québec, Canada © Hui Han, 2018 Substrate inhibition of 17 beta-hydroxysteroid dehydrogenase type 1 in living cells and regulation among the steroid-converting enzymes in breast cancers Thèse Hui Han Sous la direction de : Sheng-xiang Lin, directeur de recherche RÉSUMÉ Cette étude a permis de démontrer les fonctions et les mécanismes de la 17bêta- hydroxystéroïde déshydrogénase de type 1 (17β-HSD1) et de la stéroïde sulfatase (STS) au niveau du cancer du sein, y compris la cinétique moléculaire et cellulaire, la liaison du ligand étudiée par la titration de fluorescence, la régulation des stéroïdes et la régulation mutuelle entre les enzymes stéroïdiennes et les cellules cancéreuses du sein. 1), L’inhibition de la 17β-HSD1 par son substrat a été démontrée par la cinétique enzymatique au niveau cellulaire pour la première fois, soutenant ainsi la fonction biologique de l’inhibition produite par le substrat. 2), En tant qu’inhibiteur, la dihydrotestostérone (DHT) n’a pas affecté la concentration du substrat estrone (E1) à laquelle l’activité enzymatique a commencé à diminuer, mais certaines augmentations de vitesse ont été observées, suggérant une diminution significative de l’inhibition par le substrat. 3), Les résultats de la modulation de l’ARNm ont démontré que la transcription du gène codant la 17β-HSD7 diminuait en réponse à l’inhibition de la 17β-HSD1 ou au knockdown dans les cellules du cancer du sein par la modification estradiol (E2). -

Guidelines for the Management of Sexually Transmitted Infections
GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS 3. TREATMENT OF SPECIFIC INFECTIONS 3.1. GONOCOCCAL INFECTIONS A large proportion of gonococcal isolates worldwide are now resistant to penicillins, tetracyclines, and other older antimicrobial agents, which can therefore no longer be 32 recommended for the treatment of gonorrhoea. TREATMENT OF SPECIFIC INFECTIONS SPECIFIC OF TREATMENT It is important to monitor local in vitro susceptibility,as well as the clinical efficacy of recommended regimens. Note In general it is recommended that concurrent anti-chlamydia therapy be given to all patients with gonorrhoea, as described in the section on chlamydia infections, since dual infection is common.This does not apply to patients in whom a specific diagnosis of C. trachomatis has been excluded by a laboratory test. UNCOMPLICATED ANOGENITAL INFECTION Recommended regimens I ciprofloxacin, 500 mg orally,as a single dose OR I azithromycin, 2 g orally,as a single dose OR I ceftriaxone, 125 mg by intramuscular injection, as a single dose OR I cefixime, 400 mg orally,as a single dose OR I spectinomycin, 2 g by intramuscular injection, as a single dose. Note I Ciprofloxacin is contraindicated in pregnancy.The manufacturer does not recommend it for use in children and adolescents. GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS I There is accumulating evidence that the cure rate of Azithromycin for gonococcal infections is best achieved by a 2-gram single dose regime.The 1-gram dose provides protracted sub-therapeutic levels which may precipitate the emergence of resistance. There are variations in the anti-gonococcal activity of individual quinolones, and it is important to use only the most active. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Estriol Therapy for Autoimmune and Neurodegenerative Diseases and Disorders
(19) TZZ¥Z__T (11) EP 3 045 177 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 20.07.2016 Bulletin 2016/29 A61K 31/565 (2006.01) A61K 31/566 (2006.01) A61K 31/568 (2006.01) A61K 45/06 (2006.01) (2006.01) (2006.01) (21) Application number: 16000349.7 A61K 31/785 A61P 25/00 (22) Date of filing: 26.09.2006 (84) Designated Contracting States: (72) Inventor: Voskuhl, Rhonda R. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Los Angeles, CA 90024 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (74) Representative: Müller-Boré & Partner Patentanwälte PartG mbB (30) Priority: 26.09.2005 US 720972 P Friedenheimer Brücke 21 26.07.2006 US 833527 P 80639 München (DE) (62) Document number(s) of the earlier application(s) in Remarks: accordance with Art. 76 EPC: This application was filed on 11-02-2016 as a 13005320.0 / 2 698 167 divisional application to the application mentioned 06815626.4 / 1 929 291 under INID code 62. (71) Applicant: The Regents of the University of California Oakland, CA 94607 (US) (54) ESTRIOL THERAPY FOR AUTOIMMUNE AND NEURODEGENERATIVE DISEASES AND DISORDERS (57) The present invention relates to a dosage from comprising estriol and glatiramer acetate polymer-1 for use in the treatment of multiple sclerosis (MS), as well as to a kit comprising estriol and glatiramer acetate polymer-1. EP 3 045 177 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 045 177 A1 Description [0001] This invention was made with Government support under Grant No. -

Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes
AE_French.1104.final 10/18/04 11:03 AM Page 890 Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Abnormal vaginal discharge: What does and does not work in treating underlying causes Linda French, MD Michigan State University, East Lansing, Mich Jennifer Horton, DO Genesys Regional Medical Center Family Practice Residency, Grand Blanc, Mich Michelle Matousek, DO Henry Ford Health System, Detroit, Mich Practice recommendations part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” ■ Treat bacterial vaginosis with oral or intravagi- (JFP 2004; 53[10]:805–814), abnormal discharge nal metronidazole or with clindamycin (SOR: is more likely to be bacterial vaginosis or no A); recurrences are common (SOR: C). pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is ■ Oral and intravaginal imidazoles are also a concern. Increasing resistance of Candida equally effective in the treatment of sp. to imidazoles is associated with indiscriminate candidiasis (SOR: A); alternate therapies use of over-the-counter products. for resistant cases have been little studied. ■ Oral metronidazole is the standard ■ BACTERIAL VAGINOSIS therapy for trichomoniasis (SOR: A). The standard treatment for bacterial vaginosis Oral tinidazole, newly available in the (BV) has been oral metronidazole (Flagyl) 500 mg US in 2004, should be used in resistant twice daily for 5 to 7 days. Intravaginal 0.75% cases (SOR: B). metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2 Oral metronidazole can cause nausea and ntifungal medications for intravaginal use abdominal pain in some patients; vaginal treat- have been available in the United States ment may be preferable for them. -

Product Monograph
PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream 0.4% w/w PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules 80 mg and terconazole Vaginal Cream 0.8% w/w Antifungal Agent Janssen Inc. 19 Green Belt Dr. Date of Revision: Toronto, Ontario M3C 1L9 April 11, 2014 www.janssen.ca Submission Control No.:171096 All trademarks used under license. © 2014 Janssen Inc. 171096 - TERAZOL - APM.doc Page 1 of 23 PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules and Cream Antifungal Agent CLINICAL PHARMACOLOGY Terconazole is a synthetic triazole antifungal agent. Terconazole is active in vitro against various strains of Candida albicans. At fungistatic concentrations terconazole inhibits the transformation of yeast cells into their mycelial form. Terconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of the fungal cell membranes. Absorption Most of an intravaginally-applied dose of terconazole (mean >60%) remains in the vaginal area. Absorption into the systemic circulation is slow and limited (<20%). Maximum plasma concentrations of terconazole occur 5 to 10 hours after application of the cream or ovule. Systemic exposure to the drug is approximately proportional to the applied dose, whether applied as the cream or ovule. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects. Distribution Terconazole is highly protein bound (94.9%) and the degree of binding is independent of drug concentration. Metabolism Systemically absorbed terconazole is extensively metabolized (>95%). Elimination Across several studies, the mean elimination half-life from plasma for unchanged terconazole ranged from 6.4 to 8.5 hours. -

TERAZOL 7(Terconazole)
TERAZOL 7(terconazole) Vaginal Cream 0.4% TERAZOL 3(terconazole) Vaginal Cream 0.8% TERAZOL® 3 (terconazole) Vaginal Suppositories 80 mg DESCRIPTION TERAZOL® 7(terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3- dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Cream 0.8% is a white to off-white, water washable cream for intravaginal administration containing 0.8% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3- dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Suppositories are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis- 1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4- yl]methoxy]phenyl]-4-isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole. The structural formula of terconazole is as follows: TERCONAZOLE C26H31Cl2N5O3 [INSERT STRUCTURE HERE] Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. -

INTERRELATIONSHIPS of the ESTROGEN-PRODUCING ENZYMES NETWORK in BREAST CANCER DISSERTATION Presented in Partial Fulfillment Of
INTERRELATIONSHIPS OF THE ESTROGEN-PRODUCING ENZYMES NETWORK IN BREAST CANCER DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Wendy L. Rich, M.S. ∗∗∗∗∗ The Ohio State University 2009 Dissertation Committee: Approved by Pui-Kai Li, Ph.D., Adviser Robert W. Brueggemeier, Ph.D. Karl A. Werbovetz, Ph.D. Adviser Graduate Program in Pharmacy Charles L. Shapiro M.D. ABSTRACT In the United States, breast cancer is the most common non-skin malignancy and the second leading cause of cancer-related death in women. However, earlier detection and new, more effective treatments may be responsible for the decrease in overall death rates. Approximately 60% of breast tumors are estrogen receptor (ER) positive and thus their cellular growth is hormone-dependent. Elevated levels of estrogens, even in post- menopausal women, have been implicated in the development and progression of hormone-dependent breast cancer. Hormone therapies seek to inhibit local estrogen action and biosynthesis, which can be produced by pathways utilizing the enzymes aromatase or steroid sulfatase (STS). Cyclooxygenase-2 (COX-2), typically involved in inflammation processes, is a major regulator of aromatase expression in breast cancer cells. STS, COX-2, and aromatase are critical for estrogen biosynthesis and have been shown to be over-expressed in breast cancer. While there continues to be extensive study and successful design of potent aromatase inhibitors, much remains unclear about the regulation of STS and the clinical applications for its selective inhibition. Further studies exploring the relationships of STS with COX-2 and aromatase enzymes will aid in the understanding of its role in cancer cell growth and in the development of future hormone- dependent breast cancer therapies. -

WO 2015/054658 Al 16 April 2015 (16.04.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/054658 Al 16 April 2015 (16.04.2015) P O P C T (51) International Patent Classification: (74) Agents: PATHAK, Rahul et al; Squire Patton Boggs C07D 401/12 (2006.01) A61K 39/395 (2006.01) (US) LLP, 275 Battery Street, Suite 2600, San Francisco, C07D 409/12 (2006.01) A61K 47/48 (2006.01) California 941 11 (US). C07D 257/08 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/US20 14/060 169 AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (22) International Filing Date: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 10 October 2014 (10.10.2014) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (25) Filing Language: English KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (26) Publication Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (30) Priority Data: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 61/890,1 18 11 October 2013 ( 11. 10.2013) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -
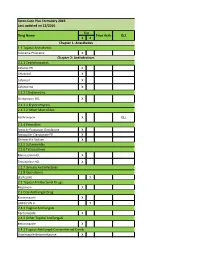
Seton-Care-Plus-2017-Formulary.Pdf
Seton Care Plus Formulary 2016 Last updated on 12/2016 Tier Drug Name Prior Auth QLL 1 2 Chapter 1: Anesthetics 1.1 Topical Anesthetics Lidocaine-Prilocaine X Chapter 2: Antiinfectives 2.1.1 Cephalosporins Cefaclor ER X Cefadroxil X Cefprozil X Cefuroxime X 2.1.2 Clindamycins Clindamycin HCL X 2.1.3.1 Erythromycins 2.1.3.2 Other Macrolides Azithromycin X QLL 2.1.4 Penicillins Amox tr-Potassium Clavulanate X Amoxicillin-Clavulanate ER X Dicloxacillin Sodium X 2.1.5 Sufonamides 2.1.6 Tetracyclines Minocycline HCL X Tetracycline HCL X 2.1.7 Urinary Antiinfectives 2.1.8 Quinolones LEVAQUIN X 2.2 Topical Antibacterial Drugs Mupirocin X 2.3 Oral Antifungal Drug Ketoconazole X GRIFULVIN V X 2.4.1 Vaginal Antifungals Terconazole X 2.4.2 Other Topical Antifungals Ketoconazole X 2.4.3 Topical Antifungal-Corticosteroid Comb Clotrimazole-Betamethasone X Nystatin-Triamcinolone X 2.5.1 Antiretrovirals & Protease Inhibitors VIREAD X PA 2.5.2 Other Antiviral Drugs Amantadine X Ribapak X RELENZA X PA TAMIFLU X 2.6 Topical Antiviral Drugs 2.7.1 Antituberculosis Drugs Rifampin X 2.7.2 Plasmodicides Mefloquine HCL X 2.7.3 Trichomonocides 2.8.1 Other Antiinfective Drugs Bacitracin X 2.8.2 Aminoglycosides Gentamicin Sulfate X Tobramycin Sulfate X Chapter 3: Antineoplastic/Immunosuppressant Drugs 3.1 Antineoplastic/Immunosuppressant Drugs Anagrelide HCL X Anastrozole X Azathioprine X PA Hydroxyurea X Leflunomide X Leucovorin Calcium X Megestrol Acetate X Mercaptopurine X Methotrexate X Tamoxifen Citrate X Tretinoin X PA Chapter 4: Cardiovascular Medications -

United States Patent 19 11 Patent Number: 5,446,070 Mantelle (45) Date of Patent: "Aug
USOO544607OA United States Patent 19 11 Patent Number: 5,446,070 Mantelle (45) Date of Patent: "Aug. 29, 1995 54 COMPOST ONS AND METHODS FOR 4,659,714 4/1987 Watt-Smith ......................... 514/260 TOPCAL ADMNSTRATION OF 4,675,009 6/1987 Hymes .......... ... 604/304 PHARMACEUTICALLY ACTIVE AGENTS 4,695,465 9/1987 Kigasawa .............................. 424/19 4,748,022 5/1988 Busciglio. ... 424/195 75 Inventor: Juan A. Mantelle, Miami, Fla. 4,765,983 8/1988 Takayanagi. ... 424/434 4,789,667 12/1988 Makino ............ ... 514/16 73) Assignee: Nover Pharmaceuticals, Inc., Miami, 4,867,970 9/1989 Newsham et al. ... 424/435 Fla. 4,888,354 12/1989 Chang .............. ... 514/424 4,894,232 1/1990 Reul ............. ... 424/439 * Notice: The portion of the term of this patent 4,900,552 2/1990 Sanvordeker .... ... 424/422 subsequent to Aug. 10, 2010 has been 4,900,554 2/1990 Yanagibashi. ... 424/448 disclaimed. 4,937,078 6/1990 Mezei........... ... 424/450 Appl. No.: 112,330 4,940,587 7/1990 Jenkins ..... ... 424/480 21 4,981,875 l/1991 Leusner ... ... 514/774 22 Filed: Aug. 27, 1993 5,023,082 6/1991 Friedman . ... 424/426 5,234,957 8/1993 Mantelle ........................... 514/772.6 Related U.S. Application Data FOREIGN PATENT DOCUMENTS 63 Continuation-in-part of PCT/US92/01730, Feb. 27, 0002425 6/1979 European Pat. Off. 1992, which is a continuation-in-part of Ser. No. 0139127 5/1985 European Pat. Off. 813,196, Dec. 23, 1991, Pat. No. 5,234,957, which is a 0159168 10/1985 European Pat.