(12) Patent Application Publication (10) Pub. No.: US 2007/0249546A1 Sawaya (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaginal Delivery System
(19) & (11) EP 2 062 568 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 27.05.2009 Bulletin 2009/22 A61K 9/00 (2006.01) (21) Application number: 07397042.8 (22) Date of filing: 22.11.2007 (84) Designated Contracting States: • Hanes, Vladimir AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Tarrytown, NY 10591 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE • Keinänen, Antti SI SK TR 20540 Turku (FI) Designated Extension States: • Holmberg, Svante AL BA HR MK RS 20900 Turku (FI) • Nikander, Hannu (71) Applicant: Bayer Schering Pharma Oy 21330 Paattinen (FI) 20210 Turku (FI) (74) Representative: Matilainen, Mirja Helena et al (72) Inventors: Oy Jalo Ant-Wuorinen AB, • Talling, Christine Iso Roobertinkatu 4-6 A 20610 Turku (FI) 00120 Helsinki (FI) (54) Vaginal delivery system (57) The present invention is related to an intravag- brane (3) encasing the core, said core and membrane inal delivery system for the controlled release of a pro- essentially consisting of a same or different polymer com- gestogen and an estrogen, comprising additionally a position, wherein at cast one of the cores comprises a therapeutically active or a health-promoting substance progestogen or a mixture of a progestogen and an es- (1) capable of giving and/or enhancing the protection trogen, and another core may comprise an estrogen or against bacterial and fungal infections, and/or enhancing a progestogen, and wherein the membrane or the surface the protection against sexually transmitted diseases. The of the membrane or at least one of the cores comprises delivery system consists of one or more compartments said therapeutically active or a health-promoting sub- (2,4,5), one of each comprising a core (7) and a mem- stance. -

Emerging Threat in Antifungal Resistance on Superficial Dermatophyte Infection R Sultana1, M Wahiduzzaman2
Bang Med J Khulna 2018; 51 : 21-24 ORIGINAL ARTICLE Emerging threat in antifungal resistance on superficial dermatophyte infection R Sultana1, M Wahiduzzaman2 Abstract Background: Dermatophytosis are most common fungul infection globally and according to WHO the prevalence is about 20-25% and does not spare people of any race or age. Over the past few years antifungal resistance has been emerged due to irrational use of antifungal drugs in cutaneous mycosis. Objective: The aim of the study is to evaluate the efficacy of different antifungul drugs (Terbinafin. Fluconazole, Itraconazole, Griseofulvin) on superficial mycosis depending on various factors. Methods: This prospective study was conducted among the Superficial fungul infected patients from April' 2017 to October 2017 in Khulna Medical College Hospital (KMCH) and dermatologist's private chamber in Khulna city. All the enrolled patients were put on oral Terbinafin, Fluconazole, Itraconazole and Griseofulvin. Each patient was given single antifungal drug orally. These cases were thus followed up after two months of treatment to look for persistent infection, cure or any relapse clinically. Result: Among 194 patient 89 were given Tab. Terbinafin (250mg) where resistance cases were 20.22%. More cases (33.96%) were resistant to Cap. Fluconazol (50mg). High percentage of cases were resistant to Cap. Itraconazole (76.47%). Griseofulvin resistant cases were observed in 25.71%. Drug response is very poor (69%) in patient who had been suffering from diabetes mellitus. Conclusion: Appropriate antifungal drugs should be chosen with strict indication, dose, duration, selection of perfect local preparation and taking laboratory facilities where necessary. Keywords : Dermatophytosis, Antifungal resistance, Public health. -

Guidelines for the Management of Sexually Transmitted Infections
GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS 3. TREATMENT OF SPECIFIC INFECTIONS 3.1. GONOCOCCAL INFECTIONS A large proportion of gonococcal isolates worldwide are now resistant to penicillins, tetracyclines, and other older antimicrobial agents, which can therefore no longer be 32 recommended for the treatment of gonorrhoea. TREATMENT OF SPECIFIC INFECTIONS SPECIFIC OF TREATMENT It is important to monitor local in vitro susceptibility,as well as the clinical efficacy of recommended regimens. Note In general it is recommended that concurrent anti-chlamydia therapy be given to all patients with gonorrhoea, as described in the section on chlamydia infections, since dual infection is common.This does not apply to patients in whom a specific diagnosis of C. trachomatis has been excluded by a laboratory test. UNCOMPLICATED ANOGENITAL INFECTION Recommended regimens I ciprofloxacin, 500 mg orally,as a single dose OR I azithromycin, 2 g orally,as a single dose OR I ceftriaxone, 125 mg by intramuscular injection, as a single dose OR I cefixime, 400 mg orally,as a single dose OR I spectinomycin, 2 g by intramuscular injection, as a single dose. Note I Ciprofloxacin is contraindicated in pregnancy.The manufacturer does not recommend it for use in children and adolescents. GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS I There is accumulating evidence that the cure rate of Azithromycin for gonococcal infections is best achieved by a 2-gram single dose regime.The 1-gram dose provides protracted sub-therapeutic levels which may precipitate the emergence of resistance. There are variations in the anti-gonococcal activity of individual quinolones, and it is important to use only the most active. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes
AE_French.1104.final 10/18/04 11:03 AM Page 890 Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Abnormal vaginal discharge: What does and does not work in treating underlying causes Linda French, MD Michigan State University, East Lansing, Mich Jennifer Horton, DO Genesys Regional Medical Center Family Practice Residency, Grand Blanc, Mich Michelle Matousek, DO Henry Ford Health System, Detroit, Mich Practice recommendations part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” ■ Treat bacterial vaginosis with oral or intravagi- (JFP 2004; 53[10]:805–814), abnormal discharge nal metronidazole or with clindamycin (SOR: is more likely to be bacterial vaginosis or no A); recurrences are common (SOR: C). pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is ■ Oral and intravaginal imidazoles are also a concern. Increasing resistance of Candida equally effective in the treatment of sp. to imidazoles is associated with indiscriminate candidiasis (SOR: A); alternate therapies use of over-the-counter products. for resistant cases have been little studied. ■ Oral metronidazole is the standard ■ BACTERIAL VAGINOSIS therapy for trichomoniasis (SOR: A). The standard treatment for bacterial vaginosis Oral tinidazole, newly available in the (BV) has been oral metronidazole (Flagyl) 500 mg US in 2004, should be used in resistant twice daily for 5 to 7 days. Intravaginal 0.75% cases (SOR: B). metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2 Oral metronidazole can cause nausea and ntifungal medications for intravaginal use abdominal pain in some patients; vaginal treat- have been available in the United States ment may be preferable for them. -

Product Monograph
PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream 0.4% w/w PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules 80 mg and terconazole Vaginal Cream 0.8% w/w Antifungal Agent Janssen Inc. 19 Green Belt Dr. Date of Revision: Toronto, Ontario M3C 1L9 April 11, 2014 www.janssen.ca Submission Control No.:171096 All trademarks used under license. © 2014 Janssen Inc. 171096 - TERAZOL - APM.doc Page 1 of 23 PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules and Cream Antifungal Agent CLINICAL PHARMACOLOGY Terconazole is a synthetic triazole antifungal agent. Terconazole is active in vitro against various strains of Candida albicans. At fungistatic concentrations terconazole inhibits the transformation of yeast cells into their mycelial form. Terconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of the fungal cell membranes. Absorption Most of an intravaginally-applied dose of terconazole (mean >60%) remains in the vaginal area. Absorption into the systemic circulation is slow and limited (<20%). Maximum plasma concentrations of terconazole occur 5 to 10 hours after application of the cream or ovule. Systemic exposure to the drug is approximately proportional to the applied dose, whether applied as the cream or ovule. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects. Distribution Terconazole is highly protein bound (94.9%) and the degree of binding is independent of drug concentration. Metabolism Systemically absorbed terconazole is extensively metabolized (>95%). Elimination Across several studies, the mean elimination half-life from plasma for unchanged terconazole ranged from 6.4 to 8.5 hours. -

TERAZOL 7(Terconazole)
TERAZOL 7(terconazole) Vaginal Cream 0.4% TERAZOL 3(terconazole) Vaginal Cream 0.8% TERAZOL® 3 (terconazole) Vaginal Suppositories 80 mg DESCRIPTION TERAZOL® 7(terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3- dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Cream 0.8% is a white to off-white, water washable cream for intravaginal administration containing 0.8% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3- dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Suppositories are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis- 1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4- yl]methoxy]phenyl]-4-isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole. The structural formula of terconazole is as follows: TERCONAZOLE C26H31Cl2N5O3 [INSERT STRUCTURE HERE] Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. -
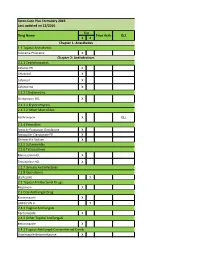
Seton-Care-Plus-2017-Formulary.Pdf
Seton Care Plus Formulary 2016 Last updated on 12/2016 Tier Drug Name Prior Auth QLL 1 2 Chapter 1: Anesthetics 1.1 Topical Anesthetics Lidocaine-Prilocaine X Chapter 2: Antiinfectives 2.1.1 Cephalosporins Cefaclor ER X Cefadroxil X Cefprozil X Cefuroxime X 2.1.2 Clindamycins Clindamycin HCL X 2.1.3.1 Erythromycins 2.1.3.2 Other Macrolides Azithromycin X QLL 2.1.4 Penicillins Amox tr-Potassium Clavulanate X Amoxicillin-Clavulanate ER X Dicloxacillin Sodium X 2.1.5 Sufonamides 2.1.6 Tetracyclines Minocycline HCL X Tetracycline HCL X 2.1.7 Urinary Antiinfectives 2.1.8 Quinolones LEVAQUIN X 2.2 Topical Antibacterial Drugs Mupirocin X 2.3 Oral Antifungal Drug Ketoconazole X GRIFULVIN V X 2.4.1 Vaginal Antifungals Terconazole X 2.4.2 Other Topical Antifungals Ketoconazole X 2.4.3 Topical Antifungal-Corticosteroid Comb Clotrimazole-Betamethasone X Nystatin-Triamcinolone X 2.5.1 Antiretrovirals & Protease Inhibitors VIREAD X PA 2.5.2 Other Antiviral Drugs Amantadine X Ribapak X RELENZA X PA TAMIFLU X 2.6 Topical Antiviral Drugs 2.7.1 Antituberculosis Drugs Rifampin X 2.7.2 Plasmodicides Mefloquine HCL X 2.7.3 Trichomonocides 2.8.1 Other Antiinfective Drugs Bacitracin X 2.8.2 Aminoglycosides Gentamicin Sulfate X Tobramycin Sulfate X Chapter 3: Antineoplastic/Immunosuppressant Drugs 3.1 Antineoplastic/Immunosuppressant Drugs Anagrelide HCL X Anastrozole X Azathioprine X PA Hydroxyurea X Leflunomide X Leucovorin Calcium X Megestrol Acetate X Mercaptopurine X Methotrexate X Tamoxifen Citrate X Tretinoin X PA Chapter 4: Cardiovascular Medications -

FAMIS Formulary (Current)
VIRGINIA PREMIER HEALTH PLAN FAMIS MEDICAID FORMULARY PLEASE NOTE: Check your benefit materials for the specific drugs covered and the copayments for your prescription drug program. For specific questions about your coverage, please call the phone number printed on your ID card. The list may not be all- inclusive. THIS LIST IS SUBJECT TO CHANGE. For the member: Generic medications contain the same active ingredients as their corresponding brand-name medications, although they may look different in color or shape. They have been FDA-approved under strict standards. For the physician: Please prescribe preferred products and allow generic substitutions when medically appropriate. Thank you. CURRENT AS OF 9/1/2021 Requirements/Limits AGE = Age limit requirement Custom = Drug has unique restrictions OTC = Over the Counter PA = Prior Authorization required QL = Quantity Level Limit lowercase italics = Generic Formulary SP = Specialty Drug. Preferred Specialty drugs Pharmacy will be listed if applicable UPPERCASE = Brand name Formulary Formulary Status ST = Step Therapy may apply to some or all drugs Formulary = Covered strengths of the drug Drug Name Formulary Status Requirements/Limits 1st relief spray external liquid 4-1 % Formulary OTC 1st tier unifine pentips 29g x 12mm , 31g x 5 mm , 31g x 6 Formulary OTC; QL (200 EA per 30 days) mm , 31g x 8 mm , 32g x 4 mm 1st tier unilet comfortouch Formulary OTC; QL (150 EA per 30 days) 4-N-1 EXTERNAL CREAM 1 % Formulary OTC 50+ adult eye health oral capsule Formulary OTC A & D ZINC OXIDE EXTERNAL -

What's Going on Down There?
What’s Going on Down There? Denise Rizzolo, PhD, PA-C Introduction • Vulvovaginitis is inflammation of the vulva and vaginal tissues. • Characterized by vaginal discharge and/or vulvar itching and irritation as well as possible vaginal odor. • Accounts for 10 million visits yearly in the US and is the most common gynecologic complaint in prepubertal girls. History- What should you ask? • Pruritus -General or just one spot • Soreness: stinging / burning / pain • Difficulty with sex • Lumps • Discharge • Partner’s have any symptoms • History of similar symptoms Physical Examination • Careful gynecologic exam • Inspection of discharge • Close examination of vulvovaginal area • Careful inspection of cervix • Look at perineum as well Physiologic Discharge • Responsible for 10 percent of cases of vaginal discharge. • Composed of vaginal squamous cells suspended in fluid medium. • Clinical characteristics: • clear to slightly cloudy • non-homogeneous • highly viscous • Changes throughout the month Normal Vaginal Discharge • Not associated with: • itching • burning • malodor • Normal increase in volume • ovulation • following coitus • after menses • during pregnancy The Big 3 •Three most common causes of vulvovaginitis include: • Bacterial Vaginosis • Vaginal candidiasis • Trichomonas Vaginalis •Others include: atrophic vaginitis, irritant vaginitis, and other STIs. Vaginal Candidiasis- Overview • Less common in postmenopausal women, unless taking estrogen. • 90% of yeast infections are secondary to Candida Albican (Most common). • Risk Factors -

Terconazole Vaginal Suppositories, 80 Mg Rx Only
TERCONAZOLE- terconazole suppository Perrigo New York Inc ---------- Terconazole Vaginal Suppositories, 80 mg Rx Only DESCRIPTION Terconazole Vaginal Suppositories, 80 mg are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)- 2- (1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4- isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole. The structural formula of terconazole is as follows: Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol. CLINICAL PHARMACOLOGY Absorption - Following a single intravaginal application of a suppository containing 240 mg 14C- terconazole to healthy women, approximately 70% (range: 64-76%) of terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5-16%) of the administered radioactivity was absorbed systemically over 7 days. Maximum plasma concentrations of terconazole occur 5 to 10 hours after intravaginal application of the suppository. Systemic exposure to terconazole is approximately proportional to the applied dose. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects. Distribution - Terconazole is highly protein bound (94.9%) in human plasma and the degree of binding is independent of drug concentration over the range of 0.01 to 5.0 mcg/mL. Metabolism - Systemically absorbed terconazole is extensively metabolized (>95%). -

TERAZOL 7 VAGINAL CREAM 0.4% (Terconazole)
TERAZOL® 7 VAGINAL CREAM 0.4% (terconazole) TERAZOL® 3 VAGINAL CREAM 0.8% (terconazole) TERAZOL® 3 VAGINAL SUPPOSITORIES 80 MG (terconazole) DESCRIPTION TERAZOL® 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1 ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Cream 0.8% is a white to off-white, water washable cream for intravaginal administration containing 0.8% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1 ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. TERAZOL® 3 (terconazole) Vaginal Suppositories are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1 ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole. The structural formula of terconazole is as follows: 1 Reference ID: 3598383 Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47.