TERAZOL 7 VAGINAL CREAM 0.4% (Terconazole)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

Vaginal Delivery System
(19) & (11) EP 2 062 568 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 27.05.2009 Bulletin 2009/22 A61K 9/00 (2006.01) (21) Application number: 07397042.8 (22) Date of filing: 22.11.2007 (84) Designated Contracting States: • Hanes, Vladimir AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Tarrytown, NY 10591 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE • Keinänen, Antti SI SK TR 20540 Turku (FI) Designated Extension States: • Holmberg, Svante AL BA HR MK RS 20900 Turku (FI) • Nikander, Hannu (71) Applicant: Bayer Schering Pharma Oy 21330 Paattinen (FI) 20210 Turku (FI) (74) Representative: Matilainen, Mirja Helena et al (72) Inventors: Oy Jalo Ant-Wuorinen AB, • Talling, Christine Iso Roobertinkatu 4-6 A 20610 Turku (FI) 00120 Helsinki (FI) (54) Vaginal delivery system (57) The present invention is related to an intravag- brane (3) encasing the core, said core and membrane inal delivery system for the controlled release of a pro- essentially consisting of a same or different polymer com- gestogen and an estrogen, comprising additionally a position, wherein at cast one of the cores comprises a therapeutically active or a health-promoting substance progestogen or a mixture of a progestogen and an es- (1) capable of giving and/or enhancing the protection trogen, and another core may comprise an estrogen or against bacterial and fungal infections, and/or enhancing a progestogen, and wherein the membrane or the surface the protection against sexually transmitted diseases. The of the membrane or at least one of the cores comprises delivery system consists of one or more compartments said therapeutically active or a health-promoting sub- (2,4,5), one of each comprising a core (7) and a mem- stance. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -

Emerging Threat in Antifungal Resistance on Superficial Dermatophyte Infection R Sultana1, M Wahiduzzaman2
Bang Med J Khulna 2018; 51 : 21-24 ORIGINAL ARTICLE Emerging threat in antifungal resistance on superficial dermatophyte infection R Sultana1, M Wahiduzzaman2 Abstract Background: Dermatophytosis are most common fungul infection globally and according to WHO the prevalence is about 20-25% and does not spare people of any race or age. Over the past few years antifungal resistance has been emerged due to irrational use of antifungal drugs in cutaneous mycosis. Objective: The aim of the study is to evaluate the efficacy of different antifungul drugs (Terbinafin. Fluconazole, Itraconazole, Griseofulvin) on superficial mycosis depending on various factors. Methods: This prospective study was conducted among the Superficial fungul infected patients from April' 2017 to October 2017 in Khulna Medical College Hospital (KMCH) and dermatologist's private chamber in Khulna city. All the enrolled patients were put on oral Terbinafin, Fluconazole, Itraconazole and Griseofulvin. Each patient was given single antifungal drug orally. These cases were thus followed up after two months of treatment to look for persistent infection, cure or any relapse clinically. Result: Among 194 patient 89 were given Tab. Terbinafin (250mg) where resistance cases were 20.22%. More cases (33.96%) were resistant to Cap. Fluconazol (50mg). High percentage of cases were resistant to Cap. Itraconazole (76.47%). Griseofulvin resistant cases were observed in 25.71%. Drug response is very poor (69%) in patient who had been suffering from diabetes mellitus. Conclusion: Appropriate antifungal drugs should be chosen with strict indication, dose, duration, selection of perfect local preparation and taking laboratory facilities where necessary. Keywords : Dermatophytosis, Antifungal resistance, Public health. -
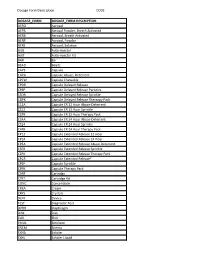
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Guidelines for the Management of Sexually Transmitted Infections
GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS 3. TREATMENT OF SPECIFIC INFECTIONS 3.1. GONOCOCCAL INFECTIONS A large proportion of gonococcal isolates worldwide are now resistant to penicillins, tetracyclines, and other older antimicrobial agents, which can therefore no longer be 32 recommended for the treatment of gonorrhoea. TREATMENT OF SPECIFIC INFECTIONS SPECIFIC OF TREATMENT It is important to monitor local in vitro susceptibility,as well as the clinical efficacy of recommended regimens. Note In general it is recommended that concurrent anti-chlamydia therapy be given to all patients with gonorrhoea, as described in the section on chlamydia infections, since dual infection is common.This does not apply to patients in whom a specific diagnosis of C. trachomatis has been excluded by a laboratory test. UNCOMPLICATED ANOGENITAL INFECTION Recommended regimens I ciprofloxacin, 500 mg orally,as a single dose OR I azithromycin, 2 g orally,as a single dose OR I ceftriaxone, 125 mg by intramuscular injection, as a single dose OR I cefixime, 400 mg orally,as a single dose OR I spectinomycin, 2 g by intramuscular injection, as a single dose. Note I Ciprofloxacin is contraindicated in pregnancy.The manufacturer does not recommend it for use in children and adolescents. GUIDELINES FOR THE MANAGEMENT OF SEXUALLY TRANSMITTED INFECTIONS I There is accumulating evidence that the cure rate of Azithromycin for gonococcal infections is best achieved by a 2-gram single dose regime.The 1-gram dose provides protracted sub-therapeutic levels which may precipitate the emergence of resistance. There are variations in the anti-gonococcal activity of individual quinolones, and it is important to use only the most active. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes
AE_French.1104.final 10/18/04 11:03 AM Page 890 Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Abnormal vaginal discharge: What does and does not work in treating underlying causes Linda French, MD Michigan State University, East Lansing, Mich Jennifer Horton, DO Genesys Regional Medical Center Family Practice Residency, Grand Blanc, Mich Michelle Matousek, DO Henry Ford Health System, Detroit, Mich Practice recommendations part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” ■ Treat bacterial vaginosis with oral or intravagi- (JFP 2004; 53[10]:805–814), abnormal discharge nal metronidazole or with clindamycin (SOR: is more likely to be bacterial vaginosis or no A); recurrences are common (SOR: C). pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is ■ Oral and intravaginal imidazoles are also a concern. Increasing resistance of Candida equally effective in the treatment of sp. to imidazoles is associated with indiscriminate candidiasis (SOR: A); alternate therapies use of over-the-counter products. for resistant cases have been little studied. ■ Oral metronidazole is the standard ■ BACTERIAL VAGINOSIS therapy for trichomoniasis (SOR: A). The standard treatment for bacterial vaginosis Oral tinidazole, newly available in the (BV) has been oral metronidazole (Flagyl) 500 mg US in 2004, should be used in resistant twice daily for 5 to 7 days. Intravaginal 0.75% cases (SOR: B). metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2 Oral metronidazole can cause nausea and ntifungal medications for intravaginal use abdominal pain in some patients; vaginal treat- have been available in the United States ment may be preferable for them. -

Over-The-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form
Keep this catalog for future orders. Over-the-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form Get OTC items delivered to your doorstep at no additional cost! NationsOTC.com Save Money with Your OTC Beneit Your OTC beneit allows you to purchase medications, health and wellness items, and irst aid supplies with home delivery at no additional cost. Ordering OTC Products is Simple 1 Choose Your Ordering Method Select the option that is best for you: ONLINE Visit NationsOTC.com and place an order. PHONE Call 833-SHOP-OTC (833-746-7682) TTY: 711 and speak with a Member Experience Advisor Monday-Friday, 8:00 a.m.-8:00 p.m. ET. Language support services are available, if needed. MAIL Complete the order form included in this catalog and mail to NationsOTC. 2 Select Your Products Choose products sorted by category. 3 Place Your Order Receive your delivery within 2-5 business days after your order is processed. If you have any questions or need help placing your order, we’re here for you. Member Experience Advisors are available at 833-SHOP-OTC (833-746-7682) TTY: 711 to make sure you get the items you need. 2 Keep this catalog to reference for future orders. Helpful Information Your Beneit Coverage Beneit Usage: This beneit is intended for members only. CMS does not allow this beneit to be used for your family or friends. Disenrollment: If you disenroll from your health plan, your OTC beneit will automatically end. _______________________________________________________________________________ Ordering Your OTC Products Products: Available over-the-counter items include health and wellness products that do not require a prescription. -

PEG Base S.A
Formulation Record Name: Progesterone Suppositories Strength: 200 mg Dosage Form: Suppository Route of Administration: Vaginal Date of Last Review or Revision: Today Person Completing Last Review or Revision: RPS Formula: Ingredient Quantity Physical Description Solubility Therapeutic Activity progesterone 200 mg/supp white, crystalline powder insoluble in water; soluble hormone replacement in alcohol silica gel 35.0 mg/supp white, amorphous powder insoluble in water; soluble suspending agent in hot alkaline hydroxides PEG Base s.a. Water miscible semisolid suppository base Example Calculations: Weight of suppository with only PEG Base = 2.1 gm Weight of progesterone in each suppository = 0.2 gm Density Factor of progesterone in PEG Base = 0.92 For making 10 suppositories (calculations based on 12 for anticipated loss of material): Weight of progesterone needed = 0.2 g × 12 = 2.4 g Weight of PEG needed if plain suppository = 2.1 g × 12 = 25.2 g Weight of PEG displaced: (weight of progesterone)/(density factor) = 2.4 g/0.92 = 2.6 g Weight of PEG base to weigh = 25.2 g - 2.6 g = 22.6 g Weight of Silica Gel to weigh = 35 mg × 12 = 420 mg (Note: displacement by silica gel is ignored) PEG base is 40% PEG 300 and 60% PEG 3350. Density of PEG 300 = 1.13. Weight of PEG base to weigh = 22.6 g Weight of PEG 300: 22.6 g × 0.4 = 9.0 g or 8.0 ml Weight of PEG 3350: 22.6 g × 0.6 = 13.6 g The shaded lines will need to be calculated based on the suppository weight in the individual molds. -

Product Monograph
PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream 0.4% w/w PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules 80 mg and terconazole Vaginal Cream 0.8% w/w Antifungal Agent Janssen Inc. 19 Green Belt Dr. Date of Revision: Toronto, Ontario M3C 1L9 April 11, 2014 www.janssen.ca Submission Control No.:171096 All trademarks used under license. © 2014 Janssen Inc. 171096 - TERAZOL - APM.doc Page 1 of 23 PRODUCT MONOGRAPH PrTERAZOL® 7 terconazole Vaginal Cream PrTERAZOL® 3 DUAL-PAK® terconazole Vaginal Ovules and Cream Antifungal Agent CLINICAL PHARMACOLOGY Terconazole is a synthetic triazole antifungal agent. Terconazole is active in vitro against various strains of Candida albicans. At fungistatic concentrations terconazole inhibits the transformation of yeast cells into their mycelial form. Terconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of the fungal cell membranes. Absorption Most of an intravaginally-applied dose of terconazole (mean >60%) remains in the vaginal area. Absorption into the systemic circulation is slow and limited (<20%). Maximum plasma concentrations of terconazole occur 5 to 10 hours after application of the cream or ovule. Systemic exposure to the drug is approximately proportional to the applied dose, whether applied as the cream or ovule. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects. Distribution Terconazole is highly protein bound (94.9%) and the degree of binding is independent of drug concentration. Metabolism Systemically absorbed terconazole is extensively metabolized (>95%). Elimination Across several studies, the mean elimination half-life from plasma for unchanged terconazole ranged from 6.4 to 8.5 hours.