PEG Base S.A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -
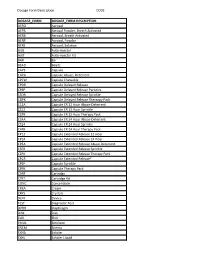
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Over-The-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form
Keep this catalog for future orders. Over-the-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form Get OTC items delivered to your doorstep at no additional cost! NationsOTC.com Save Money with Your OTC Beneit Your OTC beneit allows you to purchase medications, health and wellness items, and irst aid supplies with home delivery at no additional cost. Ordering OTC Products is Simple 1 Choose Your Ordering Method Select the option that is best for you: ONLINE Visit NationsOTC.com and place an order. PHONE Call 833-SHOP-OTC (833-746-7682) TTY: 711 and speak with a Member Experience Advisor Monday-Friday, 8:00 a.m.-8:00 p.m. ET. Language support services are available, if needed. MAIL Complete the order form included in this catalog and mail to NationsOTC. 2 Select Your Products Choose products sorted by category. 3 Place Your Order Receive your delivery within 2-5 business days after your order is processed. If you have any questions or need help placing your order, we’re here for you. Member Experience Advisors are available at 833-SHOP-OTC (833-746-7682) TTY: 711 to make sure you get the items you need. 2 Keep this catalog to reference for future orders. Helpful Information Your Beneit Coverage Beneit Usage: This beneit is intended for members only. CMS does not allow this beneit to be used for your family or friends. Disenrollment: If you disenroll from your health plan, your OTC beneit will automatically end. _______________________________________________________________________________ Ordering Your OTC Products Products: Available over-the-counter items include health and wellness products that do not require a prescription. -

2021 Formulary Annual Notice of Change
Updated: January 1, 2021 2021 Formulary Annual Notice of Change Commercial 3-Tier Plans This is a listing of the changes that have occurred to the 2021 Commercial 3-Tier formulary. For a complete list, please refer to our website and review the 2021 Commercial Comprehensive Formulary (Drug List). Please carefully review these changes. If you have any questions or need to obtain updated coverage determination and exception information, please call Customer Service toll-free at 1.855.443.4735 (TTY/TDD relay: 1.800.955.8771) Monday through Friday from 8 a.m. to 6 p.m. or visit myHFHP.org. This information is not a complete description of benefits. Contact the plan for more information. Limitations, copayments, and restrictions may apply. Benefits and copayments/co-insurance may change on January 1 of each year. You must generally use network pharmacies to use your prescription drug benefit. The Formulary and pharmacy network may change at any time. You will receive notice when necessary. Health First Commercial Plans, Inc. is doing business under the name of Health First Health Plans. Health First Health Plans does not discriminate on the basis of race, color, national origin, disability, age, sex, gender identity, sexual orientation, or health status in the administration of the plan, including enrollment and benefit determinations. 36194_MPINFO8809(10/2020) Effective Date:1/1/2021 Medication Name Change Description AIMOVIG AUTOINJECTOR 140 MG/ML Formulary Addition SUBCUTANEOUS AUTO-INJECTOR AIMOVIG AUTOINJECTOR 70 MG/ML Formulary Addition -

Rectal Corticosteroids Versus Alternative Treatments in Ulcerative Colitis: a Meta-Analysis Gut: First Published As 10.1136/Gut.40.6.775 on 1 June 1997
Gut 1997; 40: 775-781 775 Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis Gut: first published as 10.1136/gut.40.6.775 on 1 June 1997. Downloaded from J K Marshall, E J Irvine Abstract medication consistently to the splenic Background-Clear strategies to optimise flexure,49 and a larger volume seems to allow the use of corticosteroids in ulcerative more proximal delivery.10 11 Rectal foam dis- colitis are lacking. seminates medication to the rectum and distal Aim-A meta-analysis was undertaken to descending colon,'2-16 whereas suppositories examine critically the role of rectal corti- coat only the rectum.'7 18 costeroids in the management of active Although studies of rectally administered distal ulcerative colitis. corticosteroids have reported fewer systemic Methods-AJl reported randomised con- adverse effects than with oral preparations, trolled trials were retrieved by searching plasma concentrations of prednisolone were the Medline and EMBASE databases and similar after administration of identical oral or the bibliographies of relevant studies. rectal doses.'9 20 Suppression of the hypo- Trials which met inclusion criteria were thalamic-pituitary-adrenal axis in association assessed for scientific rigour. Data were with rectal therapy has also been shown.2' 26 extracted by two independent observers Newer topically active corticosteroids such according to predetermined criteria. as tixocortol, beclomethasone, prednisolone Results-Of 83 trials retrieved, 33 met metasulphabenzoate, and budesonide, with inclusion criteria. Pooled odds ratios restricted absorption or rapid hepatic metab- (POR) showed conventional rectal corti- olism have been developed to reduce the costeroids and rectal budesonide to be adverse effects associated with conventional clearly superior to placebo. -

Adverse Events: the Role of Formulations and Delivery Systems
12 Adverse events: the role of formulations and delivery systems 12.1 Introduction 482 12.11 Reactions to impurities 494 12.2 Excipient effects 483 12.12 Crystallisation 503 12.3 E-numbers 485 12.13 Abnormal bioavailability and adverse events 504 12.4 Cross-reactivity of drugs 487 12.14 Photochemical reactions and 12.5 Non-ionic surfactants 487 photoinduced reactions 506 12.6 Polyoxyethylene glycols 488 12.15 Conclusions 509 12.7 Adjuvants as therapeutic substances 488 References 510 12.8 Active excipients in multiple therapies 490 Further reading 511 12.9 Influence of dosage form type 491 12.10 Tear films and eye drops 492 The main purpose of formulations is to deliver active substances in accurate doses in medicines that are stable and of a high and consistent quality. Drugs are often the smallest proportion of a medicine, and the variety of other ingredients within most dose forms is large. Some of these excipients serve more than one purpose and some have some biological activity. The reason we discuss in this textbook adverse events which may follow from administration of medicines is that some of the problems that arise are the result of excipients interacting physically or chemically with drugs or with themselves, or creating instability or, in some instances, having biological activity of their own. That activity may not be a direct pharmacological or toxicological action, though some agents, such as some non-ionic surfactants, may cause anaphylactic responses or affect drug absorption rates and extent by solubilisation effects or by acting on P-glycoprotein receptors, thus influencing bioavailability. -

Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: a Recent Update
polymers Review Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update Manisha Pandey 1,2,* , Hira Choudhury 1,2,*, Azila Abdul-Aziz 3 , Subrat Kumar Bhattamisra 4, Bapi Gorain 5,6, Teng Carine 7 , Tan Wee Toong 7, Ngiam Jing Yi 7 and Lim Win Yi 7 1 Department of Pharmaceutical Technology, School of Pharmacy, International Medical University, Bukit Jalil, Kuala Lumpur 57000, Malaysia 2 Centre for Bioactive Molecules and Drug Delivery, Institute for Research, Development and Innovation, International Medical University, Kuala Lumpur 57000, Malaysia 3 Department of Chemical and Environmental Engineering, Malaysia-Japan International Institute of Technology, Universiti Teknologi Malaysia, Jalan Sultan Yahya Petra, Kuala Lumpur 54100, Malaysia; [email protected] or [email protected] 4 Department of Life Sciences, School of Pharmacy, International Medical University, Bukit Jalil, Kuala Lumpur 57000, Malaysia; [email protected] 5 Faculty of Health and Medical Sciences, School of Pharmacy, Taylor’s University, Subang Jaya, Selangor 47500, Malaysia; [email protected] 6 Center for Drug Delivery and Molecular Pharmacology, Faculty of Health and Medical Sciences, Taylor’s University, Subang Jaya, Selangor 47500, Malaysia 7 Undergraduate School of Pharmacy, International Medical University, Bukit Jalil, Kuala Lumpur 57000, Malaysia; [email protected] (T.C.); [email protected] (T.W.T.); [email protected] (N.J.Y.); [email protected] (L.W.Y.) * Correspondence: [email protected] or [email protected] (M.P.); [email protected] (H.C.); Tel.: +60-166-048-589 (M.P.); +60-183-830-420 (H.C.) Abstract: An optimal host–microbiota interaction in the human vagina governs the reproductive Citation: Pandey, M.; Choudhury, H.; health status of a woman. -

Achievements in Thermosensitive Gelling Systems for Rectal Administration
International Journal of Molecular Sciences Review Achievements in Thermosensitive Gelling Systems for Rectal Administration Maria Bialik, Marzena Kuras, Marcin Sobczak and Ewa Oledzka * Department of Biomaterials Chemistry, Chair of Analytical Chemistry and Biomaterials, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha St., 02-097 Warsaw, Poland; [email protected] (M.B.); [email protected] (M.K.); [email protected] (M.S.) * Correspondence: [email protected]; Tel.: +48-22-572-07-55 Abstract: Rectal drug delivery is an effective alternative to oral and parenteral treatments. This route allows for both local and systemic drug therapy. Traditional rectal dosage formulations have histori- cally been used for localised treatments, including laxatives, hemorrhoid therapy and antipyretics. However, this form of drug dosage often feels alien and uncomfortable to a patient, encouraging refusal. The limitations of conventional solid suppositories can be overcome by creating a thermosen- sitive liquid suppository. Unfortunately, there are currently only a few studies describing their use in therapy. However, recent trends indicate an increase in the development of this modern therapeutic system. This review introduces a novel rectal drug delivery system with the goal of summarising recent developments in thermosensitive liquid suppositories for analgesic, anticancer, antiemetic, antihypertensive, psychiatric, antiallergic, anaesthetic, antimalarial drugs and insulin. The report also presents the impact of various types of components and their concentration on the properties of this rectal dosage form. Further research into such formulations is certainly needed in order to meet Citation: Bialik, M.; Kuras, M.; the high demand for modern, efficient rectal gelling systems. Continued research and development Sobczak, M.; Oledzka, E. -

Compounding for Vulvar Conditions
Multidisciplinary Evaluation and Treatment of Vulvar Disorders The Role of Compounding Pharmacy Tara Thompson PharmD., RPh. Innovation Compounding Pharmacy Disclosures • Innovation Compounding Pharmacy – Kennesaw, GA Objectives • To understand the purpose of a compounding pharmacy • To familiarize the benefits & drawbacks of compounding • To delineate compounding options for the vulvar region • To determine appropriate formulations for commonly prescribed vulvar preparations What is Compounding? • Compounding pharmacists work directly with prescribers including physicians, NP/PAs, physical/sex therapists, and other clinicians to create customized medication solutions for patients whose healthcare needs cannot be met by manufactured medications. • 5000 year old profession, the oldest form of pharmacy • Regulated by State Boards of Pharmacy and Accreditation Boards • “Thinking outside of the box” • Manipulating the dosage form! • Customized, individualized medicine tailored to the patient’s specific need How Do I Choose a Compounding Pharmacy? • Licensed in your State • Pharmacist Knowledge and Access • Adherence to USP Guidelines, Testing • USP <795> - Non-Sterile Compounding • USP <797> - Sterile Compounding • Diversity of Portfolio to your Practice Needs • Pelvic Health/FSD/IC • Pricing and Patient Care/Access • Reputation • Compounding Ability and Dosage Form Assortment • Most Important – Accreditation and Inspection!! PCAB – Pharmacy Compounding Accreditation Board • A service of the Accreditation Commission for Healthcare (ACHC) • -

WO 2018/090022 A2 17 May 2018 (17.05.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/090022 A2 17 May 2018 (17.05.2018) W !P O PCT (51) International Patent Classification: A61K 36/185 (2006.01) A61K 31/4045 (2006.01) (21) International Application Number: PCT/US2017/061584 (22) International Filing Date: 14 November 2017 (14.1 1.2017) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/421,933 14 November 2016 (14. 11.2016) US (71) Applicant: FARM TO FARMA, INC. [US/US]; PO Box 8880, Incline Village, Nevada 89450 (US). (72) Inventor: CROWLEY, Kenton L.; 40970 Alton Court, Temecula, California 92591 (US). (74) Agent: BORING, Landin F. et al; Mailstop: IP Docketing - 22, 1100 Peachtree Street, Suite 2800, Atlanta, Georgia 30309 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW.