WO 2018/090022 A2 17 May 2018 (17.05.2018) W !P O PCT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide
Human Journals Research Article September 2015 Vol.:4, Issue:2 © All rights are reserved by C. Aparna et al. Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide Keywords: Nateglinide, transdermal patch and gel, HPMC, ethyl cellulose, carbopol, PVA, PVP ABSTRACT Anusha Gundeti, C. Aparna*, Dr. Prathima Srinivas The objective of the present work was to formulate Transdermal Drug Delivery systems of Nateglinide, an Department of Pharmaceutics, Sri Venkateshwara antidiabetic drug belonging to meglitinide class with a half life of 1.5 hrs. Transdermal patches containing nateglinide were College of Pharmacy, prepared by solvent casting method using the combinations Affiliated to Osmania University, of HPMC:EC, PVA:PVP, HPMC:Eudragit RS 100, Eudragit RL100:RS100 in different proportions and by incorporating Madhapur, Hyderabad, Telangana -500081, India. different permeation enhancers (polyethylene glycol 400, Su bmission: 7 September 2015 DMSO). The transdermal patches were evaluated for their physicochemical properties like thickness, weight variation, Accepted: 11 September 2015 folding endurance, percentage moisture absorption, percentage Published: 25 September 2015 moisture loss, in-vitro diffusion studies & ex-vivo permeation studies. Transdermal Gel was formulated using HPMC, carbopol 934, carbopol 940 and methyl cellulose. Gels were evaluated for homogeneity, pH, viscosity, drug content, in-vitro diffusion studies & ex-vivo permeation studies. By comparing the drug release F5 (HPMC:EC) formulation was selected as optimized formulation as it could sustain the drug release for 12 hrs i.e. 99.2% when compared to gel. Stability studies were www.ijppr.humanjournals.com carried out according to ICH guidelines and the patches maintained integrity and good physicochemical properties during the study period. -

Transdermal Absorption Preparation
Europäisches Patentamt *EP001522316A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 316 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: A61K 47/34, A61K 47/10, 13.04.2005 Bulletin 2005/15 A61K 47/14, A61K 9/06, A61K 9/08, A61K 9/12, (21) Application number: 03764126.3 A61K 9/70 (22) Date of filing: 02.07.2003 (86) International application number: PCT/JP2003/008400 (87) International publication number: WO 2004/006960 (22.01.2004 Gazette 2004/04) (84) Designated Contracting States: • OMICHI, Katsuhiro AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Saitama-shi, Saitama 338-0832 (JP) HU IE IT LI LU MC NL PT RO SE SI SK TR • OKADA, Minoru Designated Extension States: Inzai-shi, Chiba 270-1323 (JP) AL LT LV MK • KURAZUMI, Toshiaki Narita-shi, Chiba 286-0011 (JP) (30) Priority: 16.07.2002 JP 2002206565 (74) Representative: Hartz, Nikolai F., Dr. (71) Applicant: SSP Co., Ltd. Wächtershäuser & Hartz Chuo-ku, Tokyo 103-8481 (JP) Patentanwälte Weinstrasse 8 (72) Inventors: 80333 München (DE) • NARUI, Takashi Sakura-shi, Chiba 285-0817 (JP) (54) TRANSDERMAL ABSORPTION PREPARATION (57) A transdermal absorption promotion composi- and transdermal absorption preparation not only exhibit tion comprising the following components (a), (b), and an excellent transdermal absorption promotion effect, (c) and a transdermal absorption preparation compris- but also exhibit superior skin-permeability, even if a drug ing the following components (a), (b), (c), and (d) are having a relatively high lipophilic property and poor disclosed. -

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -

An Archetype Swing in Transdermal Drug Delivery
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 A COMPREHENSIVE REVIEW ON MICRONEEDLES - AN ARCHETYPE SWING IN TRANSDERMAL DRUG DELIVERY G. Ravi*, N. Vishal Gupta, M. P. Gowrav Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Shri Shivarathreeshwara Nagara, Mysuru, Karnataka, India. ARTICLE INFO ABSTRACT Article history Transdermal drug delivery is the non-invasive delivery of medications through the skin Received 23/12/2016 surface into the systemic circulation. The advantage of transdermal drug delivery system is Available online that it is painless technique of administration of drugs. The advantage of transdermal drug 31/01/2017 delivery system is that it is painless technique of administration of drugs. Transdermal drug delivery system can improve the therapeutic efficacy and safety of the drugs because drug Keywords delivered through the skin at a predetermined and controlled rate. Due to the various Microneedles, biomedical benefits, it has attracted many researches. The barrier nature of stratumcorneum Hypodermic Needles, poses a danger to the drug delivery. By using microneedles, a pathway into the human body Transdermal, can be recognized which allow transportation of macromolecular drugs such as insulin or Stratumcorneum, vaccine. These microneedles only penetrate outer layers of the skin, exterior sufficient not to Patch. reach the nerve receptors of the deeper skin. Thus the microneedles supplement is supposed painless and reduces the infection and injuries. Researches from the past few years showed that microneedles have emerged as a novel carrier and considered to be effective for safe and improved delivery of the different drugs. Microneedles development is created a new pathway in the drug delivery field. -

The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome As Transdermal Delivery Carriers
Send Orders for Reprints to [email protected] The Open Conference Proceedings Journal, 2013, 4, 87-92 87 Open Access The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome as Transdermal Delivery Carriers Sureewan Duangjit1,2, Praneet Opanasopit1, Theerasak Rojarata1, Yasuko Obata2, Yoshinori Oniki2, Kozo Takayama2 and Tanasait Ngawhirunpat1,* 1Department of Pharmaceutical Technology, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand 2Department of Pharmaceutics, Hoshi University, Ebara 2-4-41, Shinagawa-ku, Tokyo 142-8501, Japan Abstract: The role of deformable liposomes characteristics on skin permeability has evoked considerable interest, since the articles reporting the effectiveness of transfersomes for skin delivery were increasingly published. Several reports focus on the effect of formulation factor which directly affected the transfersome’s skin permeability. However, the effect of formulation factors was not fully understood as the contradictory results. To clarify this problem, the reliable statistical techniques, excellent experimental design and systematical variation were used in this study. Transfersomes loaded meloxicam containing controlled amount of phosphatidylcholine (PC), cholesterol (Chol), type of surfactant (hydrophilic part, lipophilic part ) were prepared and investigated for the physicochemical characteristics (e.g., size, size distribution, charge, elasticity, drug content, morphology) and skin permeability. The results indicated -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery. -
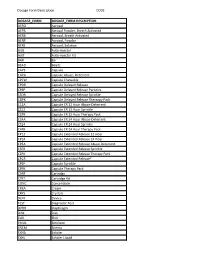
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Transdermal Nicotine Maintenance Attenuates the Subjective And
Neuropsychopharmacology (2004) 29, 991–1003 & 2004 Nature Publishing Group All rights reserved 0893-133X/04 $25.00 www.neuropsychopharmacology.org Transdermal Nicotine Maintenance Attenuates the Subjective and Reinforcing Effects of Intravenous Nicotine, but not Cocaine or Caffeine, in Cigarette-Smoking Stimulant Abusers 1 1 ,1,2 Bai-Fang X Sobel , Stacey C Sigmon and Roland R Griffiths* 1Department of Psychiatry and Behavioral Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA The effects of transdermal nicotine maintenance on the subjective, reinforcing, and cardiovascular effects of intravenously administered cocaine, caffeine, and nicotine were examined using double-blind procedures in nine volunteers with histories of using tobacco, caffeine, and cocaine. Each participant was exposed to two chronic drug maintenance phases (21 mg/day nicotine transdermal patch and placebo transdermal patch). Within each drug phase, the participant received intravenous injections of placebo, cocaine (15 and 30 mg/70 kg), caffeine (200 and 400 mg/70 kg), and nicotine (1.0 and 2.0 mg/70 kg) in mixed order across days. Subjective and cardiovascular data were collected before and repeatedly after drug or placebo injection. Reinforcing effects were also assessed after each injection with a Drug vs Money Multiple-Choice Form. Intravenous cocaine produced robust dose-related increases in subjective and reinforcing effects; these effects were not altered by nicotine maintenance. Intravenous caffeine produced elevations on several subjective ratings; nicotine maintenance did not affect these ratings. Under the placebo maintenance condition, intravenous nicotine produced robust dose-related subjective effects, with maximal increases similar to the high dose of cocaine; nicotine maintenance significantly decreased the subjective and reinforcing effects of intravenous nicotine. -

A Brief Review on Transdermal Patches
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 7 Issue 2 - June 2018 Copyright © All rights are reserved by Nidhi Sharma DOI: 10.19080/OMCIJ.2018.07.555707 A Brief Review on Transdermal Patches Nidhi Sharma* HIMT College of Pharmacy, Greater Noida, India Submission: May 12, 2018; Published: June 05, 2018 *Corresponding author: Nidhi Sharma, HIMT College of Pharmacy, Greater Noida, India, Email: Abstract healingTransdermal to an injured drug area delivery of the system body. was An presentedadvantage toof overcome a transdermal the difficulties drug delivery of drug route delivery over especiallyother types oral of route. delivery A transdermal system such patch as oral, is a topical,medicated i.v., adhesive i.m., etc. ispatch that that the patchis placed provides on the askin controlled to deliver release a specific of the dose medication of medication into the through patient, the usually skin and through into the either bloodstream. a porous It membrane promotes covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier, as a result, only medications whose molecules are small can easily penetrate the skin, so it can be delivered by this method. This review article describes the overall introduction of transdermal patches including type of transdermal patches, method of preparation of transdermal -

Over-The-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form
Keep this catalog for future orders. Over-the-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form Get OTC items delivered to your doorstep at no additional cost! NationsOTC.com Save Money with Your OTC Beneit Your OTC beneit allows you to purchase medications, health and wellness items, and irst aid supplies with home delivery at no additional cost. Ordering OTC Products is Simple 1 Choose Your Ordering Method Select the option that is best for you: ONLINE Visit NationsOTC.com and place an order. PHONE Call 833-SHOP-OTC (833-746-7682) TTY: 711 and speak with a Member Experience Advisor Monday-Friday, 8:00 a.m.-8:00 p.m. ET. Language support services are available, if needed. MAIL Complete the order form included in this catalog and mail to NationsOTC. 2 Select Your Products Choose products sorted by category. 3 Place Your Order Receive your delivery within 2-5 business days after your order is processed. If you have any questions or need help placing your order, we’re here for you. Member Experience Advisors are available at 833-SHOP-OTC (833-746-7682) TTY: 711 to make sure you get the items you need. 2 Keep this catalog to reference for future orders. Helpful Information Your Beneit Coverage Beneit Usage: This beneit is intended for members only. CMS does not allow this beneit to be used for your family or friends. Disenrollment: If you disenroll from your health plan, your OTC beneit will automatically end. _______________________________________________________________________________ Ordering Your OTC Products Products: Available over-the-counter items include health and wellness products that do not require a prescription.