Compounding for Vulvar Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Leucoplakic Vulva: Premalignant Determinants C
Henry Ford Hospital Medical Journal Volume 11 | Number 3 Article 3 9-1963 The Leucoplakic Vulva: Premalignant Determinants C. Paul Hodgkinson Roy B. P. Patton M. A. Ayers Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Hodgkinson, C. Paul; Patton, Roy B. P.; and Ayers, M. A. (1963) "The Leucoplakic Vulva: Premalignant Determinants," Henry Ford Hospital Medical Bulletin : Vol. 11 : No. 3 , 279-287. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol11/iss3/3 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. For more information, please contact [email protected]. Henry Ford Hosp. Med. Bull. Vol. 11, September, 1963 THE LEUCOPLAKIC VULVA Premalignant Determinants C. PAUL HODGKINSON, M.D.,* ROY B. P. PATTON, M.D., AND M. A. AYERS, M.D.* IN A PAPER proposing to discuss the leucoplakic vulva and any predisposing ten dency it may have to the development of squamous cell carcinoma, the term "pre malignant" has presumptuous connotations. This is presumptuous because it implies that more is known about cancer and its mode of development than can be supported by facts. What happens in the cell prior to the stage of carcinoma-in-situ is a burning and unsolved question in cancer research. How to detect and appraise the parameters of malignant potential is the essence of meaning connoted by the word "premalignant". -

Baclofen Overdose D
Postgraduate Medical Journal (February 1980) 56, 108-109 Postgrad Med J: first published as 10.1136/pgmj.56.652.108 on 1 February 1980. Downloaded from Baclofen overdose D. J. LIPSCOMB* T. J. MEREDITHt M.B.. M.R.C.P. M.A., M.R.C.P. *Department of Medicine, Peterborough District Hospital, Peterborough PE3 6DA, and tPoisons Unit, Guy's Hospital, London SE] 9RT Summary Case report A 57-year-old woman suffering from multiple sclerosis A 51-year-old woman, who had suffered from took an estimated 1500 mg of baclofen. She became multiple sclerosis for 15 years, was prescribed baclo- deeply unconscious with generalized flaccid muscle fen 40 mg daily as part of her treatment for spastic paralysis and absent tendon reflexes. Toxicological paraplegia. One morning, she was found lying in analysis confirmed the presence of baclofen together bed unconscious and was admitted to hospital. with small amounts of paracetamol and glutethimide. Information given by her husband suggested that an Supportive therapy, including assisted ventilation for overdose of baclofen (consisting of 152 10-mg 3 days, led to complete recovery; anticonvulsant tablets) had been taken about 2 5 hr before being drugs were necessary for the treatment of grand mal found unconscious. On arrival in hospital 90 min fits. The clinical features and treatment of baclofen later, she was deeply unconscious and unresponsive overdose are discussed. to painful stimuli. Respiration was depressed and the cough reflex was absent. She was intubated without Introduction resistance and ventilated; gastric lavage was per-Protected by copyright. Baclofen (Lioresal, Ciba) is widely used in the formed but no tablets were returned in the lavage treatment of muscle spasticity. -

The Use of Intravenous Baclofen As Therapy for the Γ-Hydroxybutyric Acid Withdrawal Syndrome
Research Article Remedy Open Access Published: 12 Jun, 2017 The Use of Intravenous Baclofen as Therapy for the γ-hydroxybutyric Acid Withdrawal Syndrome Marc Sabbe1*, Francis Desmet1 and Sabrina Dewinter2 1Department of Emergency Medicine, University Hospitals, Catholic University of Leuven, Belgium 2Department of Pharmacy, University Hospitals, Catholic University of Leuven, Belgium Abstract Introduction: In this case series with three patients, we introduced baclofen, a γ-aminobutyric acid type B(GABA-B) receptor agonist, for treatment of the γ-hydroxybutyric acid (GHB) withdrawal syndrome. Materials and Methods: Single center case series performed on three patientswith a GHB withdrawal syndrome. They initially received massive doses of benzodiazepines, without sufficient effect. Two patients also received an unsuccessful continuous dexmedetomidine drip. In all patients, intravenous baclofen was started, with an intravenous loading dose between 0.5 and 2 milligrams (mg) to achieve a therapeutic level. Thereafter a continuous intravenous dose between 0.5 and 1 mg per hour for 12 h was administered to maintain a steady state. After that, baclofen was substituted orally with a daily oral dose varying between 20 mg and 40 mg which could be downgraded and stopped over the next days. They all continued to receive a standard benzodiazepine regimen during the baclofen trial. Results and Discussion: Main outcome measurements were the degree of withdrawal symptoms and the need for benzodiazepines during baclofen treatment. In all patients, a significant reduction of the GHB withdrawal syndrome was noted. A standard daily regimen of baseline benzodiazepine dosing between 40 mg and 80 mg diazepam was sufficient. Adverse effects of baclofen use were absent. -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Vaginitis and Abnormal Vaginal Bleeding
UCSF Family Medicine Board Review 2013 Vaginitis and Abnormal • There are no relevant financial relationships with any commercial Vaginal Bleeding interests to disclose Michael Policar, MD, MPH Professor of Ob, Gyn, and Repro Sciences UCSF School of Medicine [email protected] Vulvovaginal Symptoms: CDC 2010: Trichomoniasis Differential Diagnosis Screening and Testing Category Condition • Screening indications – Infections Vaginal trichomoniasis (VT) HIV positive women: annually – Bacterial vaginosis (BV) Consider if “at risk”: new/multiple sex partners, history of STI, inconsistent condom use, sex work, IDU Vulvovaginal candidiasis (VVC) • Newer assays Skin Conditions Fungal vulvitis (candida, tinea) – Rapid antigen test: sensitivity, specificity vs. wet mount Contact dermatitis (irritant, allergic) – Aptima TMA T. vaginalis Analyte Specific Reagent (ASR) Vulvar dermatoses (LS, LP, LSC) • Other testing situations – Vulvar intraepithelial neoplasia (VIN) Suspect trich but NaCl slide neg culture or newer assays – Psychogenic Physiologic, psychogenic Pap with trich confirm if low risk • Consider retesting 3 months after treatment Trichomoniasis: Laboratory Tests CDC 2010: Vaginal Trichomoniasis Treatment Test Sensitivity Specificity Cost Comment Aptima TMA +4 (98%) +3 (98%) $$$ NAAT (like GC/Ct) • Recommended regimen Culture +3 (83%) +4 (100%) $$$ Not in most labs – Metronidazole 2 grams PO single dose Point of care – Tinidazole 2 grams PO single dose •Affirm VP III +3 +4 $$$ DNA probe • Alternative regimen (preferred for HIV infected -

Localised Provoked Vestibulodynia (Vulvodynia): Assessment and Management
FOCUS Localised provoked vestibulodynia (vulvodynia): assessment and management Helen Henzell, Karen Berzins Background hronic vulvar pain (pain lasting more than 3–6 months, but often years) is common. It is estimated to affect 4–8% of Vulvodynia is a chronic vulvar pain condition. Localised C women at any one time and 10–20% in their lifetime.1–3 provoked vestibulodynia (LPV) is the most common subset Little attention has been paid to the teaching of this condition of vulvodynia, the hallmark symptom being pain on vaginal so medical practitioners may not recognise the symptoms, and penetration. Young women are predominantly affected. LPV diagnosis is often delayed.2 Community awareness is low, but is a hidden condition that often results in distress and shame, increasing with media attention. Women can be confused by the is frequently unrecognised, and women usually see a number symptoms and not know how to discuss vulvar pain. The onus is of health professionals before being diagnosed, which adds to on medical practitioners to enquire about vulvar pain, particularly their distress and confusion. pain with sex, when taking a sexual or reproductive health history. Objective Vulvodynia The aim of this article is to inform health providers about the Vulvodynia is defined by the International Society for the Study assessment and management of LPV. of Vulvovaginal Disease (ISSVD) as ‘chronic vulvar discomfort, most often described as burning pain, occurring in the absence Discussion of relevant findings or a specific, clinically identifiable, neurologic 4 Diagnosis is based on history. Examination is used to support disorder’. It is diagnosed when other causes of vulvar pain have the diagnosis. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
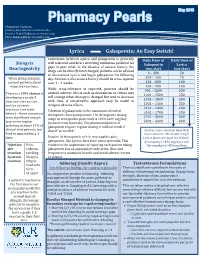
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days.