Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colitis Caused by Non-Steroidal Anti-Inflammatory Drugs
Postgrad Med J: first published as 10.1136/pgmj.62.730.773 on 1 August 1986. Downloaded from Postgraduate Medical Journal (1986) 62, 773-776 Colitis caused by non-steroidal anti-inflammatory drugs S. Ravi', A.C. Keat2 and E.C.B. Keat1 'Cuckfield Hospital, Cuckfield, West Sussex, and2Westminster Hospital, Horseferry Road, London SWIP2AP, UK. Summary: Four cases of acute proctocolitis associated with non-steroidal anti-inflammatory drug therapy are presented. The drugs implicated were flufenamic acid, mefenamic acid, naproxen and ibuprofen. After resolution of symptoms and signs of proctocolitis three of the four patients were subsequently rechallenged with the implicated drug: in each there was a rapid relapse. Introduction Ulcerative colitis is a disease of unknown aetiology Case reports with characteristic clinical features and a protracted course. A similar clinical picture, but running a shorter Case I and usually benign course, is occasionally seen follow- ing the administration of certain drugs. This was first A 77 year old woman was referred with intermittent noticed following the administration of antibiotics, bleeding per rectum for 6 months, associated for the often with pseudomembrane formation. Later, this last 2 months with bloody diarrhoea up to eight times was shown to be associated with infection by toxigenic daily. Previously, she had had troublesome symptoms Clostridium difficile. Until 1978, most cases were from osteoarthritis of her back and knees for which copyright. associated with treatment with clindamycin but since she had been prescribed flufenamic acid 200 mg thrice that time nearly all antibiotics have been implicated. daily. Her general health had remained good but she Other drugs capable of causing proctocolitis, though appeared pale and her haemoglobin was reduced to by different mechanisms, include phenindione (Keat & 8 g/dl. -

Journal of Pharmacology and Experimental Therapeutics
Journal of Pharmacology and Experimental Therapeutics Molecular Determinants of Ligand Selectivity for the Human Multidrug And Toxin Extrusion Proteins, MATE1 and MATE-2K Bethzaida Astorga, Sean Ekins, Mark Morales and Stephen H Wright Department of Physiology, University of Arizona, Tucson, AZ 85724, USA (B.A., M.M., and S.H.W.) Collaborations in Chemistry, 5616 Hilltop Needmore Road, Fuquay-Varina NC 27526, USA (S.E.) Supplemental Table 1. Compounds selected by the common features pharmacophore after searching a database of 2690 FDA approved compounds (www.collaborativedrug.com). FitValue Common Name Indication 3.93897 PYRIMETHAMINE Antimalarial 3.3167 naloxone Antidote Naloxone Hydrochloride 3.27622 DEXMEDETOMIDINE Anxiolytic 3.2407 Chlordantoin Antifungal 3.1776 NALORPHINE Antidote Nalorphine Hydrochloride 3.15108 Perfosfamide Antineoplastic 3.11759 Cinchonidine Sulfate Antimalarial Cinchonidine 3.10352 Cinchonine Sulfate Antimalarial Cinchonine 3.07469 METHOHEXITAL Anesthetic 3.06799 PROGUANIL Antimalarial PROGUANIL HYDROCHLORIDE 100MG 3.05018 TOPIRAMATE Anticonvulsant 3.04366 MIDODRINE Antihypotensive Midodrine Hydrochloride 2.98558 Chlorbetamide Antiamebic 2.98463 TRIMETHOPRIM Antibiotic Antibacterial 2.98457 ZILEUTON Antiinflammatory 2.94205 AMINOMETRADINE Diuretic 2.89284 SCOPOLAMINE Antispasmodic ScopolamineHydrobromide 2.88791 ARTICAINE Anesthetic 2.84534 RITODRINE Tocolytic 2.82357 MITOBRONITOL Antineoplastic Mitolactol 2.81033 LORAZEPAM Anxiolytic 2.74943 ETHOHEXADIOL Insecticide 2.64902 METHOXAMINE Antihypotensive Methoxamine -

Investigating the Influence of Polymers on Supersaturated
Page 1 of 45 Molecular Pharmaceutics 1 2 3 4 5 6 7 Investigating the Influence of Polymers on 8 9 10 11 12 Supersaturated Flufenamic Acid Cocrystal Solutions 13 14 15 16 1 1 2 2 1 17 Minshan Guo , Ke Wang , Noel Hamill , Keith Lorimer and Mingzhong Li * 18 19 20 1School of pharmacy, De Montfort University, Leicester, UK 21 22 23 2Almac Science, Seagoe Industrial Estate, Craigavon, UK 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 ACS Paragon Plus Environment 1 Molecular Pharmaceutics Page 2 of 45 1 2 3 4 5 6 7 Table of contents graphic 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 ACS Paragon Plus Environment 2 Page 3 of 45 Molecular Pharmaceutics 1 2 3 Abstract 4 5 6 7 The development of enabling formulations is a key stage when demonstrating the effectiveness 8 9 10 of pharmaceutical cocrystals to maximize the oral bioavailability for poorly water soluble drugs. 11 12 Inhibition of drug crystallization from a supersaturated cocrystal solution through a fundamental 13 14 understanding of the nucleation and crystal growth is important. In this study, the influence of 15 16 17 the three polymers of polyethylene glycol (PEG), polyvinylpyrrolidone (PVP) and a copolymer 18 19 of N-vinly-2-pyrrodidone (60%) and vinyl acetate (40%) (PVP-VA) on the flufenamic acid 20 21 22 (FFA) crystallization from three different supersaturated solutions of the pure FFA and two 23 24 cocrystals of FFA-NIC CO and FFA-TP CO has been investigated by measuring nucleation 25 26 induction times and desupersaturation rates in the presence and absence of seed crystals. -

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Development of Pain-Free Methods for Analyzing 231 Multiclass Drugs and Metabolites by LC-MS/MS
Clinical, Forensic & Toxicology Article “The Big Pain”: Development of Pain-Free Methods for Analyzing 231 Multiclass Drugs and Metabolites by LC-MS/MS By Sharon Lupo As the use of prescription and nonprescription drugs grows, the need for fast, accurate, and comprehensive methods is also rapidly increasing. Historically, drug testing has focused on forensic applications such as cause of death determinations or the detection of drug use in specific populations (military, workplace, probation/parole, sports doping). However, modern drug testing has expanded well into the clinical arena with a growing list of target analytes and testing purposes. Clinicians often request the analysis of large panels of drugs and metabolites that can be used to ensure compliance with prescribed pain medication regimens and to detect abuse or diversion of medications. With prescription drug abuse reaching epidemic levels [1], demand is growing for analytical methods that can ensure accurate results for comprehensive drug lists with reasonable analysis times. LC-MS/MS is an excellent technique for this work because it offers greater sensitivity and specificity than immunoassay and—with a highly selective and retentive Raptor™ Biphenyl column—can provide definitive results for a wide range of compounds. Typically, forensic and pain management drug testing consists of an initial screening analysis, which is qualitative, quick, and requires only minimal sample preparation. Samples that test positive during screening are then subjected to a quantitative confirmatory analysis. Whereas screening assays may cover a broad list of compounds and are generally less sensitive and specific, confirmation testing provides fast, targeted analysis using chromatographic conditions that are optimized for specific panels. -

Flufenamic Acid, Mefenamic Acid and Niflumic Acid Inhibit Single Nonselective Cation Channels in the Rat Exocrine Pancreas
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 268, number 1, 79-82 FEBS 08679 July 1990 Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas H. Gagelein*, D. Dahlem, H.C. Englert** and H.J. Lang** Max-Planck-Institutfir Biophysik, Kennedyallee 70, D-600 Frankfurt/Main 70, FRG Received 17 May 1990 The non-steroidal anti-inflammatory drugs, flufenamic acid, mefenamic acid and niflumic acid, block Ca2+-activated non-selective cation channels in inside-out patches from the basolateral membrane of rat exocrine pancreatic cells. Half-maximal inhibition was about 10 PM for flufenamic acid and mefenamic acid, whereas niflumic acid was less potent (IC,, about 50 PM). Indomethacin, aspirin, diltiazem and ibuprofen (100 /IM) had not effect. It is concluded that the inhibitory effect of flufenamate, mefenamate and niflumate is dependent on the specific structure, consisting of two phenyl rings linked by an amino bridge. Mefenamic acid; Flufenamic acid; Niflumic acid; Indomethacin; Non-selective cation channel; Rat exocrine pancreas 1. INTRODUCTION indomethacin, ibuprofen, diltiazem and acetylsalicylic acid (aspirin) were obtained from Sigma (Munich, FRG). The substances were dissolved in dimethylsulfoxide (DMSO, Merck, Darmstadt, FRG, Recently it was reported that the drug, 3 ’ ,5-dichloro- 0.1% of total volume) before addition to the measuring solution. diphenylamine-2-carboxylic acid (DCDPC), blocks DMSO alone had no effect on the single channel recordings. non-selective cation channels in the basolateral mem- 2.2. Methods brane of rat exocrine pancreatic cells [ 11. -

Synthesis and Pharmacological Evaluation of Fenamate Analogues: 1,3,4-Oxadiazol-2-Ones and 1,3,4- Oxadiazole-2-Thiones
Scientia Pharmaceutica (Sci. Pharm.) 71,331-356 (2003) O Osterreichische Apotheker-Verlagsgesellschaft m. b.H., Wien, Printed in Austria Synthesis and Pharmacological Evaluation of Fenamate Analogues: 1,3,4-Oxadiazol-2-ones and 1,3,4- Oxadiazole-2-thiones Aida A. ~l-~zzoun~'*,Yousreya A ~aklad',Herbert ~artsch~,~afaaA. 2aghary4, Waleed M. lbrahims, Mosaad S. ~oharned~. Pharmaceutical Sciences Dept. (Pharmaceutical Chemistry goup' and Pharmacology group2), National Research Center, Tahrir St. Dokki, Giza, Egypt. 3~nstitutflir Pharmazeutische Chemie, Pharrnazie Zentrum der Universitilt Wien. 4~harmaceuticalChemistry Dept. ,' Organic Chemistry Dept. , Helwan University , Faculty of Pharmacy, Ein Helwan Cairo, Egypt. Abstract A series of fenamate pyridyl or quinolinyl analogues of 1,3,4-oxadiazol-2-ones 5a-d and 6a-r, and 1,3,4-oxadiazole-2-thiones 5e-g and 6s-v, respectively, have been synthesized and evaluated for their analgesic (hot-plate) , antiinflammatory (carrageenin induced rat's paw edema) and ulcerogenic effects as well as plasma prostaglandin E2 (PGE2) level. The highest analgesic activity was achieved with compound 5a (0.5 ,0.6 ,0.7 mrnolkg b.wt.) in respect with mefenamic acid (0.4 mmollkg b.wt.). Compounds 6h, 61 and 5g showed 93, 88 and 84% inhibition, respectively on the carrageenan-induced rat's paw edema at dose level of O.lrnrnol/kg b.wt, compared with 58% inhibition of mefenamic acid (0.2mmoll kg b.wt.). Moreover, the highest inhibitory activity on plasma PGE2 level was displayed also with 6h, 61 and 5g (71, 70,68.5% respectively, 0.lmmolkg b.wt.) compared with indomethacin (60%, 0.01 mmolkg b.wt.) as a reference drug. -

LZI Buprenorphine Enzyme Immunoassay for in Vitro Diagnostic Use Only 8ºC 0270 (100/37.5 Ml R1/R2 Kit) 0271 (1000/375 Ml R1/R2 Kit) 2ºC
LZI Buprenorphine Enzyme Immunoassay For In Vitro Diagnostic Use Only 8ºC 0270 (100/37.5 mL R1/R2 Kit) 0271 (1000/375 mL R1/R2 Kit) 2ºC Lin-Zhi International, Inc. Intended Use Calibrators and Controls* The Lin-Zhi International (LZI) Buprenorphine Enzyme Immunoassay is *Calibrators and controls are sold separately and contain negative human intended for the qualitative and semi-quantitative determination of urine with sodium azide as a preservative. norbuprenorphine (a buprenorphine metabolite) in human urine at a cutoff value NORBUPRENORPHINE Calibrators/Controls REF of 5 ng/mL and 10 ng/mL. The assay is designed for prescription use with a Negative Calibrator 0001 number of automated clinical chemistry analyzers. Control: Contains 3 ng/mL norbuprenorphine 0272 The assay provides only a preliminary analytical result. A more specific Cutoff/Calibrator: Contains 5 ng/mL norbuprenorphine 0273 alternative analytical chemistry method must be used in order to obtain a Control: Contains 7 ng/mL norbuprenorphine 0274 confirmed analytical result. Gas or Liquid Chromatography/Mass Cutoff/Calibrator: Contains 10 ng/mL norbuprenorphine 0275 Control: Contains 13 ng/mL norbuprenorphine 0276 Spectrometry (GC/MS or LC/MS) are the preferred confirmatory methods Calibrator: Contains 20 ng/mL norbuprenorphine 0277 (1, 2). Clinical consideration and professional judgment should be exercised Calibrator: Contains 40 ng/mL norbuprenorphine 0278 with any drug of abuse test result, particularly when the preliminary test Calibrator: Contains 75 ng/mL norbuprenorphine 0279 result is positive. Precautions and Warning Summary and Explanation of Test • This test is for in vitro diagnostic use only. Harmful if swallowed. Buprenorphine is a semi-synthetic opioid derived from thebaine, an alkaloid of • Reagents used in the assay contain sodium azide as a preservative, which the poppy plant, Papaver somniferum. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
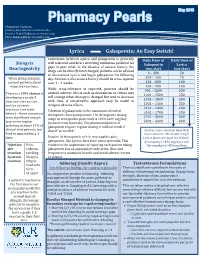
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas.