Fatal Toxicity of Antidepressant Drugs in Overdose
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stopping Antidepressants
Stopping antidepressants This information is for anyone who wants to know more about stopping antidepressants. It describes: symptoms that you may get when stopping an antidepressant some ways to reduce or avoid these symptoms. This patient information accurately reflects recommendations in the NICE guidance on depression in adults Disclaimer This resource provides information, not advice. The content in this resource is provided for general information only. It is not intended to, and does not, amount to advice which you should rely on. It is not in any way an alternative to specific advice. You must therefore obtain the relevant professional or specialist advice before taking, or refraining from, any action based on the information in this resource. If you have questions about any medical matter, you should consult your doctor or other professional healthcare provider without delay. If you think you are experiencing any medical condition, you should seek immediate medical attention from a doctor or other professional healthcare provider. Although we make reasonable efforts to compile accurate information in our resources and to update the information in our resources, we make no representations, warranties or guarantees, whether express or implied, that the content in this resource is accurate, complete or up to date. What are antidepressants? They are medications prescribed for depressive illness, anxiety disorder or obsessive- compulsive disorder (OCD). You can find out more about how they work, why they are prescribed, their effects and side-effects, and alternative treatments in our separate resource on antidepressants. Usually, you don’t need to take antidepressants for more than 6 to 12 months. -

Differential Effects on Suicidal Ideation of Mianserin, Maprotiline and Amitriptyline S
Br. J. clin. Pharmac. (1978), 5, 77S-80S DIFFERENTIAL EFFECTS ON SUICIDAL IDEATION OF MIANSERIN, MAPROTILINE AND AMITRIPTYLINE S. MONTGOMERY Academic Department of Psychiatry, Guy's Hospital Medical School, London Bridge, London SE1 9RT, UK B. CRONHOLM, MARIE ASBERG Karolinska Institute, Stockholm D.B. MONTGOMERY Institute of Psychiatry, De Crespigny Park, Denmark Hill, London SE5 8AF, U K 1 Eighty patients suffering from primary depressive illness were treated for 4 weeks, following a 7-d (at least) no-treatment period, with either mianserin 60 mg daily (n = 50), maprotiline 150 mg at night- time (n = 15) or amitriptyline 150 mg at night-time (n = 15) in double-blind controlled trials run con- currently in the same hospital on protocols having the same design and entry criteria. 2 The effects of the three drugs were examined for their proffle of action using a new depression rating scale (Montgomery and Asberg Depression Scale, MADS) claimed to be more sensitive to change. There was no significant difference in mean entry scores or mean amelioration of depression on the MADS or the Hamilton Rating Scale (HRS) between the three drugs, but individual items on the MADS did show significant differences in amelioration. A highly significant (P < 0.01) difference was observed in favour of mianserin against maprotiline on both items of'suicidal thoughts' and 'pessimistic thoughts' on the MADS. The suicidal item on the HRS showed a similar tendency which did not reach significance. 3 This finding is considered in relation to a possible mode of action of mianserin and the relevance to reported subgroups ofdepressive illness with increased suicidal ideation. -

Dose Equivalents of Antidepressants Evidence-Based Recommendations
Journal of Affective Disorders 180 (2015) 179–184 Contents lists available at ScienceDirect Journal of Affective Disorders journal homepage: www.elsevier.com/locate/jad Research report Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials Yu Hayasaka a,n, Marianna Purgato b, Laura R Magni c, Yusuke Ogawa a, Nozomi Takeshima a, Andrea Cipriani b,d, Corrado Barbui b, Stefan Leucht e, Toshi A Furukawa a a Department of Health Promotion and Human Behavior, Kyoto University Graduate School of Medicine/School of Public Health, Yoshida Konoe-cho, Sakyo- ku, Kyoto 606-8501, Japan b Department of Public Health and Community Medicine, Section of Psychiatry, University of Verona, Policlinico “G.B.Rossi”, Pzz.le L.A. Scuro, 10, Verona 37134, Italy c Psychiatric Unit, Istituto di Ricovero e Cura a Carattere Scientifico, Centro San Giovanni di Dio, Fatebenefratelli, Brescia, Italy d Department of Psychiatry, University of Oxford, Oxford, UK e Department of Psychiatry and Psychotherapy, Technische Universität München, Klinikum rechts der Isar, Ismaningerstr. 22, 81675 Munich, Germany article info abstract Article history: Background: Dose equivalence of antidepressants is critically important for clinical practice and for Received 4 February 2015 research. There are several methods to define and calculate dose equivalence but for antidepressants, Received in revised form only daily defined dose and consensus methods have been applied to date. The purpose of the present 10 March 2015 study is to examine dose equivalence of antidepressants by a less arbitrary and more systematic method. Accepted 12 March 2015 Methods: We used data from all randomized, double-blind, flexible-dose trials comparing fluoxetine or Available online 31 March 2015 paroxetine as standard drugs with any other active antidepressants as monotherapy in the acute phase Keywords: treatment of unipolar depression. -

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Strategies for Managing Sexual Dysfunction Induced by Antidepressant Medication
King’s Research Portal DOI: 10.1002/14651858.CD003382.pub3 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Taylor, M. J., Rudkin, L., Bullemor-Day, P., Lubin, J., Chukwujekwu, C., & Hawton, K. (2013). Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database of Systematic Reviews, (5). https://doi.org/10.1002/14651858.CD003382.pub3 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Development of Pain-Free Methods for Analyzing 231 Multiclass Drugs and Metabolites by LC-MS/MS
Clinical, Forensic & Toxicology Article “The Big Pain”: Development of Pain-Free Methods for Analyzing 231 Multiclass Drugs and Metabolites by LC-MS/MS By Sharon Lupo As the use of prescription and nonprescription drugs grows, the need for fast, accurate, and comprehensive methods is also rapidly increasing. Historically, drug testing has focused on forensic applications such as cause of death determinations or the detection of drug use in specific populations (military, workplace, probation/parole, sports doping). However, modern drug testing has expanded well into the clinical arena with a growing list of target analytes and testing purposes. Clinicians often request the analysis of large panels of drugs and metabolites that can be used to ensure compliance with prescribed pain medication regimens and to detect abuse or diversion of medications. With prescription drug abuse reaching epidemic levels [1], demand is growing for analytical methods that can ensure accurate results for comprehensive drug lists with reasonable analysis times. LC-MS/MS is an excellent technique for this work because it offers greater sensitivity and specificity than immunoassay and—with a highly selective and retentive Raptor™ Biphenyl column—can provide definitive results for a wide range of compounds. Typically, forensic and pain management drug testing consists of an initial screening analysis, which is qualitative, quick, and requires only minimal sample preparation. Samples that test positive during screening are then subjected to a quantitative confirmatory analysis. Whereas screening assays may cover a broad list of compounds and are generally less sensitive and specific, confirmation testing provides fast, targeted analysis using chromatographic conditions that are optimized for specific panels. -

Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW
Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW Name of Medicine / Trimipramine (Surmontil®) Class (generic and brand) Licensed indication(s) Treatment of depressive illness, especially where sleep disturbance, anxiety or agitation are presenting symptoms. Sleep disturbance is controlled within 24 hours and true antidepressant action follows within 7 to 10 days. Licensed dose(s) Adults: For depression 50-75 mg/day initially increasing to 150-300 mg/day in divided doses or one dose at night. The maintenance dose is 75-150 mg/day. Elderly: 10-25 mg three times a day initially. The initial dose should be increased with caution under close supervision. Half the normal maintenance dose may be sufficient to produce a satisfactory clinical response. Children: Not recommended. Purpose of Document To review information currently available on this class of medicines, give guidance on potential use and assign a prescribing classification http://www.cambsphn.nhs.uk/CJPG/CurrentDrugClassificationTable.aspx Annual cost (FP10) 10mg three times daily: £6,991 25mg three times daily: £7,819 150mg daily: £7,410 300mg daily: £14,820 Alternative Treatment Options within Class Tricyclic Annual Cost CPCCG Formulary Classification Antidepressant (FP10) Amitriptyline (75mg) Formulary £36 Lofepramine (140mg) Formulary £146 Imipramine (75mg) Non-formulary £37 Clomipramine (75mg) Non-formulary £63 Trimipramine (75mg). TBC £7,819 Nortriptyline (75mg) Not Recommended (pain) £276 Doxepin (150mg) TBC £6,006 Dosulepin (75mg) Not Recommended (NICE DO NOT DO) £19 Dosages are based on possible maintenance dose and are not equivalent between medications Recommendation It is recommended to Cambridgeshire and Peterborough CCG JPG members and through them to local NHS organisations that the arrangements for use of trimipramine are in line with restrictions agreed locally for drugs designated as NOT RECOMMENDED:. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -
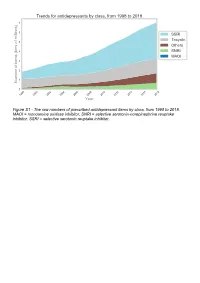
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

LUMIN Mianserin Hydrochloride Tablets
AUSTRALIAN PRODUCT INFORMATION LUMIN Mianserin hydrochloride tablets 1 NAME OF THE MEDICINE Mianserin hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Mianserin belongs to the tetracyclic series of antidepressant compounds, the piperazinoazepines. These are chemically different from the common tricyclic antidepressants. Each Lumin 10 tablet contains 10 mg of mianserin hydrochloride and each Lumin 20 tablet contains 20 mg of mianserin hydrochloride as the active ingredient. Lumin also contains trace amounts of sulfites. For the full list of excipients, see Section 6.1 List of Excipients. 3 PHARMACEUTICAL FORM Lumin 10: 10 mg tablet: white, film coated, normal convex, marked MI 10 on one side, G on reverse Lumin 20: 20 mg tablet: white, film coated, normal convex, marked MI 20 on one side, G on reverse 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS For the treatment of major depression. 4.2 DOSE AND METHOD OF ADMINISTRATION Lumin tablets should be taken orally between meals, preferably with a little fluid, and swallowed without chewing. Use in Children and Adolescents (< 18 years of age) Lumin should not be used in children and adolescents under the age of 18 years (see section 4.4 Special Warnings and Precautions for use). Adults The initial dosage of Lumin should be judged individually. It is recommended that treatment begin with a daily dose of 30 mg given in three divided doses or as a single bedtime dose and be adjusted weekly in the light of the clinical response. The effective daily dose for adult patients usually lies between 30 mg and 90 mg (average 60 mg) in divided doses or as a single bedtime dose. -

Slow Trasicor Tablets 160Mg (Oxprenolol Hydrochloride)
Prescribing information slow trasicor tablets 160mg (oxprenolol hydrochloride) Presentation: Contains 160mg oxprenolol hydrochloride in a danger of cardiac arrest, a calcium antagonist of the verapamil sustained-release tablet type must not be administered intravenously to patients already indication: Hypertension and angina pectoris (long-term receiving treatment with a beta-blocker. Beta-blockers should be prophylaxis) discontinued in patients who are at increased risk for anaphylaxis. Dosage and administration: Oral tablets to be swallowed whole. interactions: Verapamil, diltiazem, disopyramide, quinidine, Adults: Hypertension: 160mg once daily. If necessary, the dosage amiodarone, adrenaline, noradrenaline, isoprenaline, ephedrine, can be raised to 320mg. Angina pectoris: 160mg once daily. If phenylephrine, clonidine, guanethidine, reserpine, oral antidiabetic necessary, the dosage can be raised to 320mg. Elderly: No special drugs, NSAIDs, cimetidine, ergot alkaloids, anaesthetics, digitalis dosage regimen is necessary but concurrent hepatic insufficiency glycosides, lidocaine and alcohol. should be taken into account. Children: Not recommended. Pregnancy and lactation: Use with caution during pregnancy contraindications: Hypersensitivity to oxprenolol and related especially in the first 3 months. Breast feeding is not recommended. derivatives, cross-sensitivity to other beta-blockers or to any of the Undesirable effects: Very Common: Dry mouth, constipation. excipients, cardiogenic shock, second or third degree atrioventricular Common: