Preferred Drug List Drug Class Review Announcement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

Resolution of Refractory Pruritus with Aprepitant in a Patient With
Case Report Resolution of refractory pruritus with aprepitant in a patient with microcystic adnexal carcinoma Johanna S Song, MD,ab Hannah Song, BA,a Nicole G Chau, MD,ac Jeffrey F Krane, MD, PhD,ad Nicole R LeBoeuf, MD, MPHabe aHarvard Medical School; bDepartment of Dermatology, Brigham and Women’s Hospital; cCenter for Head and Neck Oncology, Dana-Farber Cancer Institute; dHead and Neck Pathology Service, Brigham and Women’s Hospital; and eCenter for Cutaneous Oncology, Dana-Farber Cancer Institute, all in Boston, Massachusetts ubstance P is an important neurotransmit- biopsied, and the patient was diagnosed with MAC ter implicated in itch pathways.1 After bind- with gross nodal involvement. Laboratory findings ing to its receptor, neurokinin-1 (NK-1), including serum chemistries, blood urea nitrogen, substance P induces release of factors including complete blood cell count, thyroid, and liver func- S 2 histamine, which may cause pruritus. Recent lit- tion were normal. Positron emission tomography- erature has reported successful use of aprepitant, an computed tomography (PET-CT) imaging was NK-1 antagonist that has been approved by the US negative for distant metastases. Food and Drug Administration for the treatment Treatment was initiated with oral aprepitant – of chemotherapy-induced nausea and vomiting, for 125 mg on day 1, 80 mg on day 2, and 80 mg on treatment of pruritus. We report here the case of a day 3 –with concomitant weekly carboplatin (AUC patient with microcystic adnexal carcinoma (MAC) 1.5) and paclitaxel (30 mg/m2) as well as radiation. who presented with refractory pruritus and who had Within hours after the first dose of aprepitant, the rapid and complete resolution of itch after adminis- patient reported a notable cessation in his pruri- tration of aprepitant. -

HHS Template for Reports, with Instructions
Texas Vendor Drug Program 1.1 Drug Use Criteria: Substance P/Neurokinin1 Receptor Antagonists Publication History 1. Developed December 2003. 2. Revised September 2020; September 2018; September 2016; May 2015; August 2013; June 2013; September 2011; October 2009; February 2006; January 2006. Notes: Information on indications for use or diagnosis is assumed to be unavailable. All criteria may be applied retrospectively; prospective application is indicated with an asterisk [*]. The information contained is for the convenience of the public. The Texas Health and Human Services Commission is not responsible for any errors in transmission or any errors or omissions in the document. Medications listed in the tables and non-FDA approved indications included in these retrospective criteria are not indicative of Vendor Drug Program formulary coverage. Prepared by: • Drug Information Service, UT Health San Antonio. • The College of Pharmacy, The University of Texas at Austin. 1 1 Dosage [*] Current therapies for chemotherapy-induced nausea/vomiting (CINV) and post- operative nausea and vomiting (PONV) target corticosteroid, dopamine, and serotonin (5-HT3) receptors. In the central nervous system, tachykinins and neurokinins play a role in some autonomic reflexes and behaviors. Aprepitant is a selective human substance P/neurokinin 1 (NK1) antagonist with a high affinity for NK1 receptors and little, if any, attraction for corticosteroid, dopamine, or 5-HT3 receptors. Rolapitant (Varubi®), the newest substance P/NK1 antagonist, is FDA- approved to prevent delayed CINV with initial and repeat chemotherapy courses including, but not limited to, highly emetogenic chemotherapy in adults. Combination therapy including netupitant, a substance P/NK1 antagonist and palonosetron, a selective 5-HT3 receptor antagonist (Akynzeo®), is now available to prevent acute and delayed CINV with initial and repeat chemotherapy courses including, but not limited to, highly emetogenic chemotherapy in adults. -

Effects of Selexipag and Its Active Metabolite in Contrasting The
Cutolo et al. Arthritis Research & Therapy (2018) 20:77 https://doi.org/10.1186/s13075-018-1577-0 RESEARCH ARTICLE Open Access Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts Maurizio Cutolo1*, Barbara Ruaro1, Paola Montagna1, Renata Brizzolara1, Emanuela Stratta2, Amelia Chiara Trombetta1, Stefano Scabini2, Pier Paolo Tavilla3, Aurora Parodi3, Claudio Corallo4, Nicola Giordano4, Sabrina Paolino1, Carmen Pizzorni1, Alberto Sulli1, Vanessa Smith5 and Stefano Soldano1 Abstract Background: Myofibroblasts contribute to fibrosis through the overproduction of extracellular matrix (ECM) proteins, primarily type I collagen (COL-1) and fibronectin (FN), a process which is mediated in systemic sclerosis (SSc) by the activation of fibrogenic intracellular signaling transduction molecules, including extracellular signal-regulated kinases 1 and 2 (Erk1/2) and protein kinase B (Akt). Selexipag is a prostacyclin receptor agonist synthesized for the treatment of pulmonary arterial hypertension. The study investigated the possibility for selexipag and its active metabolite (ACT-333679) to downregulate the profibrotic activity in primary cultures of SSc fibroblasts/myofibroblasts and the fibrogenic signaling molecules involved. Methods: Fibroblasts from skin biopsies obtained with Ethics Committee (EC) approval from patients with SSc, after giving signed informed consent, were cultured until the 3rd culture passage and then either maintained in normal growth medium (untreated cells) or independently treated with different concentrations of selexipag (from 30 μMto 0.3 μM) or ACT-333679 (from 10 μMto0.1μM) for 48 h. Protein and gene expressions of α-smooth muscle actin (α-SMA), fibroblast specific protein-1 (S100A4), COL-1, and FN were investigated by western blotting and quantitative real-time PCR. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Gastroparesis: 2014
GASTROINTESTINAL MOTILITY AND FUNCTIONAL BOWEL DISORDERS, SERIES #1 Richard W. McCallum, MD, FACP, FRACP (Aust), FACG Status of Pharmacologic Management of Gastroparesis: 2014 Richard W. McCallum Joseph Sunny, Jr. Gastroparesis is characterized by delayed gastric emptying without mechanical obstruction of the gastric outlet or small intestine. The main etiologies are diabetes, idiopathic and post- gastric and esophageal surgical settings. The management of gastroparesis is challenging due to a limited number of medications and patients often have symptoms, which are refractory to available medications. This article reviews current treatment options for gastroparesis including adverse events and limitations as well as future directions in pharmacologic research. INTRODUCTION astroparesis is a syndrome characterized by documented gastroparesis are increasing.2 Physicians delayed emptying of gastric contents without have both medical and surgical approaches for these Gmechanical obstruction of the stomach, pylorus or patients (See Figure 1). Medical therapy includes both small bowel. Patients can present with nausea, vomiting, prokinetics and antiemetics (See Table 1 and Table 2). postprandial fullness, early satiety, pressure, fullness The gastroparesis population will grow as diabetes and abdominal distension. In addition, abdominal pain increases and new therapies will be required. What located in the epigastrium, and distinguished from the do we know about the size of the gastroparetic term discomfort, is increasingly being recognized population? According to a study from the Mayo Clinic as an important symptom. The main etiologies of group surveying Olmsted County in Minnesota, the gastroparesis are diabetes, idiopathic, and post gastric risk of gastroparesis in Type 1 diabetes mellitus was and esophageal surgeries.1 Hospitalizations from significantly greater than for Type 2. -
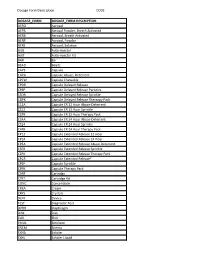
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Nanosuspensions of Selexipag: Formulation, Characterization, and in Vitro Evaluation Rusul M
Iraqi J Pharm Sci, Vol.30(1) 2021 Selexipag nanosuspension DOI : https://doi.org/10.31351/vol30iss1pp144-153 Nanosuspensions of Selexipag: Formulation, Characterization, and in vitro Evaluation Rusul M. Alwan*,1 and Nawal A. Rajab** * Ministry of Health and Environment, Najaf Health Department, Najaf, Iraq. **Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq. Abstract Selexipag(SLP) is an orally selective long-acting prostacyclin receptor agonist indicated for pulmonary arterial hypertension treatment. It is practically insoluble in water (class II, according to BCS). This work aims to prepare and optimize Selexipag nanosuspensions (SLPNS) to enhance the saturation solubility and in vitro dissolution rate. The solvent antisolvent precipitation method was used for the production of NS, and the effect of formulation parameters (stabilizer type, drug: stabilizer ratio, and use of co-stabilizer) and process parameter (stirring speed) on the particle size (P.S) and polydispersity index (PDI) were studied. The result revealed that the P.S of all prepared SLPNS formulation was in the nanometer range, except for the formulas that stabilized by Poloxamer. The optimal SLPNS (F15), which is stabilized by Soluplus® (SLP: stabilizer ratio 1:2) and prepared at a stirring speed of 1000 rpm, showed the smallest P.S and appropriate PDI, which are 47 nm and 0.073. The formula F5 exhibits 136 folds, an increase in the saturation solubility, and an enhancement in the dissolution rate in phosphate buffer pH 6.8 (100% drug release during 60 min) compared to the pure drug. This result indicates that SLPNS is an efficient way for improving the saturation solubility and the dissolution rate of SLP. -

Initial Public Comment for Aprepitant for Chemotherapy-Induced Emesis CAG-00248N July 6 – August 6, 2004
Initial Public Comment for Aprepitant for Chemotherapy-Induced Emesis CAG-00248N July 6 – August 6, 2004 Commenter: Duncan, Sariah, RN, BSN, OCN Organization: Date: August 2, 2004 Comment: Please Do Not Limit Anti-Emetic Coverage!! I don't think Emend should be considered full replacement for other covered treatments for chemotherapy induced emesis. On the few patients who it was prescribed to in our clinic, it did not always work well. We find that we still have to give the patient IV anti-emetics, even if they took Emend, because they still throw up. ------------------------------------------------------------------------------------------------------- Commenter: Takahashi, Gary Organization: Oregon Hematology Oncology Association Date: August 6, 2004 Comment: In my experience, Emend (aprepitant) is useful only as an adjunct to other antiemetics to prevent delayed-onset nausea and vomiting. It is only mildly effective when given alone, and must be combined with more potent anti-emetics to control acute-onset nausea. I recommend against using Emend as an oral substitute for drugs such as granisetron or palonosetron. -------------------------------------------------------------------------------------------------------- Commenter: D’Emanuele, Ross Organization: Dorsey & Whitney, LLP Date: August 6, 2004 Comment: Public Comment Offered in Response to CMS’ National Coverage Analysis (NCA) Titled “Aprepitant for Chemotherapy-Induced Emesis” (CAG-00248N) POSITION Oral EMEND® is not a replacement for any current commercially available intravenous antiemetic in the United States. Therefore, it is not appropriate to reimburse it as a Medicare Part B benefit. EMEND needs to be administered in conjunction with a 5-HT3 receptor antagonist and is not stand alone therapy. It does not function as a prodrug or have an IV equivalent. -

The Significance of NK1 Receptor Ligands and Their Application In
pharmaceutics Review The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy Agnieszka Majkowska-Pilip * , Paweł Krzysztof Halik and Ewa Gniazdowska Centre of Radiochemistry and Nuclear Chemistry, Institute of Nuclear Chemistry and Technology, Dorodna 16, 03-195 Warsaw, Poland * Correspondence: [email protected]; Tel.: +48-22-504-10-11 Received: 7 June 2019; Accepted: 16 August 2019; Published: 1 September 2019 Abstract: To date, our understanding of the Substance P (SP) and neurokinin 1 receptor (NK1R) system shows intricate relations between human physiology and disease occurrence or progression. Within the oncological field, overexpression of NK1R and this SP/NK1R system have been implicated in cancer cell progression and poor overall prognosis. This review focuses on providing an update on the current state of knowledge around the wide spectrum of NK1R ligands and applications of radioligands as radiopharmaceuticals. In this review, data concerning both the chemical and biological aspects of peptide and nonpeptide ligands as agonists or antagonists in classical and nuclear medicine, are presented and discussed. However, the research presented here is primarily focused on NK1R nonpeptide antagonistic ligands and the potential application of SP/NK1R system in targeted radionuclide tumour therapy. Keywords: neurokinin 1 receptor; Substance P; SP analogues; NK1R antagonists; targeted therapy; radioligands; tumour therapy; PET imaging 1. Introduction Neurokinin 1 receptor (NK1R), also known as tachykinin receptor 1 (TACR1), belongs to the tachykinin receptor subfamily of G protein-coupled receptors (GPCRs), also called seven-transmembrane domain receptors (Figure1)[ 1–3]. The human NK1 receptor structure [4] is available in Protein Data Bank (6E59).