Rectal Medications July 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

Bowel Preparation Instructions Fleet Enema
66 BOWEL PREPARATION INSTRUCTIONS FLEET ENEMA Please read these instructions before you have your enema and follow carefully. If you need more advice before using the enema, please telephone the Endoscopy Unit on Tel: 01246 512197 You have been given a phosphate enema, which should be kept and used at room temperature. This is to be used on the day you are coming to hospital for your flexible sigmoidoscopy procedure. Instructions: • The enema must be given at least one hour before you leave home to attend hospital. e.g. if your appointment is at 2.00pm and you will be leaving at 1.30pm you must give the enema at 12.30pm. • To give the enema, you need to lie down on your side with both knees bent and pulled up towards your chest. • Remove the enema cap and throw it away. • Relax and gently insert the full length of the nozzle into your rectum (back passage). • Do this without using undue force. The tip of the nozzle is already lubricated to make insertion easier • Squeeze the bottle until it is completely empty. The bottle may be rolled up (like a tube of toothpaste) ensure it empties. Then gently withdraw the bottle while still squeezing to keep it rolled up. • Hold the liquid in your back passage for as long as you can (by clenching your buttocks) 66 MAKE SURE YOU ARE CLOSE TO A TOILET AS THE ENEMA WILL RESULT IN A RAPID EMPTYING OF YOUR BOWELS • Return the used enema to its carton and throw away safely in your domestic waste. -

About Your Surgery Pre-Operative Instructions Prior to Surgery Post-Operative Instructions How to Reach Us
www.fpminstitute.com About Your Surgery Pre-operative Instructions Prior to Surgery Post-operative Instructions How to Reach Us www.fpminstitute.com Pre-operative Instructions Please Note Patient Name: Procedure: Vaginal Colpopexy Enterocele / Rectocele Repair A physician at The Institute for Female Pelvic Medicine & Sacral Colpopexy Colpocleisis / Colpectomy Reconstructive Surgery is available for emergencies 24 hours per day. Hysterectomy Removal / Revision Uterosacral Vault Suspension InterStim® Office hours are Monday through Friday, 8 a.m. to 4 p.m. Colporrhaphy Pubovaginal Sling After hours and on weekends, you can leave non-urgent messages Other: that will be returned the next business day. For urgent situations, Surgery Date: the answering service can page the physician on call. Medical Clearance Blood work at Lab Blood work on Admit. You are required to be medically cleared for surgery by your primary care physician and/or cardiologist within 30 days of your scheduled surgery. You have been given a medical clearance form that your physician will need to complete, as well as a script for an EKG and bloodwork. Please contact your physician and schedule your medical clearance appointment for the week of (not before this www.fpminstitute.com date). If your physician sends you to a lab for bloodwork, we suggest you do this at least two to three days before your appointment, but not before the date indicated above. We will need copies of your test results and completed medical clearance form faxed to us at the number on the form at least three (3) business days prior to your surgery. If you have any questions, please contact our nursing department. -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -
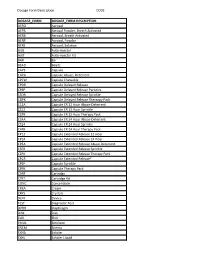
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Therapeutic Enema for Intussusception
Therapeutic Enema for Intussusception Therapeutic enema is used to help identify and diagnose intussusception, a serious disorder in which one part of the intestine slides into another in a telescoping manner and causes inflammation and an obstruction. Intussusception often occurs at the junction of the small and large intestine and most commonly occurs in children three to 24 months of age. A therapeutic enema using air or a contrast material solution may be performed to create pressure within the intestine and "un-telescope" the intussusception while relieving the obstruction. This exam is usually performed on an emergency basis. Tell your doctor about your child's recent illnesses, medical conditions, medications and allergies, especially to barium or iodinated contrast materials. Your child may be asked to wear a gown and remove any objects that might interfere with the x-ray images. An ultrasound may be performed to help confirm the diagnosis. What is a Therapeutic Enema for Intussusception? What is intussusception? Intussusception is a serious disorder in which one part of the intestine slides into another part of the intestine, similar to a collapsing telescope. The intestine becomes inflamed and swollen and can cause an obstruction or blockage. Symptoms can include severe abdominal pain, fever, vomiting or abnormal stools. Intussusception may occur anywhere along the gastrointestinal tract; however, it often occurs at the junction of the small and large intestine. The condition most commonly occurs in children three months to 24 months of age. Intussusception is a medical/surgical emergency. If your child has some or all of the symptoms of intussusception, you should call your physician or an emergency medical professional immediately. -

Comparative Study of Enema Retention and Preference in Ulcerative Colitis J R Ingram, J Rhodes, B K Evans, R G Newcombe, G a O Thomas
594 Postgrad Med J: first published as 10.1136/pgmj.2004.031690 on 2 September 2005. Downloaded from ORIGINAL ARTICLE Comparative study of enema retention and preference in ulcerative colitis J R Ingram, J Rhodes, B K Evans, R G Newcombe, G A O Thomas ............................................................................................................................... Postgrad Med J 2005;81:594–598. doi: 10.1136/pgmj.2004.031690 Background: Therapeutic enemas are often used to treat active colitis but their retention may be limited because of urgency to defecate. Some preparations may be better retained and tolerated than others See end of article for because of their physical properties. authors’ affiliations ....................... Aim: To compare patient preference and retention of four therapeutic enemas, including a nicotine enema, in patients with ulcerative colitis (UC). Correspondence to: Methods: Twenty four patients with active UC received the four trial enemas—corticosteroid, 5-amino Dr G A O Thomas, Department of salicylate (5-ASA), and nicotine liquid enemas and a corticosteroid foam, in a randomised order, taking Gastroenterology, one enema on each of four successive nights. Patients scored them 1 to 4 for ease of administration and University Hospital of retention, degree of abdominal bloating, and for their overall preference. Wales, Heath Park, Cardiff CF14 4XW, UK; Gareth. Results: Fifteen patients rated nicotine their overall favourite or second favourite, compared with 14 for [email protected]. corticosteroid foam and 11 for 5-ASA and corticosteroid liquids, but this was not significant (p = 0.302). nhs.uk Overall, there was no significant difference in overnight retention. However, the nicotine enema tended to be less well retained in patients with milder urgency but a higher proportion retained it overnight with Submitted 16 December 2004 more severe urgency (p = 0.031 compared with 5-ASA enema). -

Vaginal Support Pessaries: Indications for Use and Fitting Strategies
SERIES Vaginal Support Pessaries: Indications for Use and Fitting Strategies Shanna Atnip and Katharine O’Dell ESSARIES P elvic floor disorders are © 2012 Society of Urologic Nurses and Associates common in women, and as the population ages, Atnip, S., & O’Dell, K. (2012). Vaginal support pessaries: Indications for use and these disorders may be fitting strategies. Urologic Nursing, 32(3), 114-125. Pseen more frequently by health ERIES ON care providers (Nygaard et al., Flexible silicone vaginal support pessaries offer a low-risk, effective option for S 2008). When pelvic symptoms treatment of symptoms of pelvic organ prolapse. This first article in a three-part series summarizes clinical recommendations and current evidence related to are associated with loss of struc- pessary indications, choice, and fitting. tural support of the pelvic organs and vagina, vaginal support pes- PECIAL Key Words: Pelvic organ prolapse, pessary, indications, fitting. saries offer an important option S for relief (American College of Objectives: Obstetricians & Gynecologists 1. List the symptoms of pelvic organ prolapse that may be successfully treated [ACOG], 2007). with a vaginal support pessary. Historically, vaginal pessaries have been used to manage pelvic 2. Discuss the various types of pessaries and their uses to treat pelvic organ floor relaxation and were made prolapse. from a variety of materials, 3. Outline the pessary selection process and steps to pessary fitting. including fruit, metal, porcelain, rubber, and acrylic (Shah, Sultan, genic, and washable, and can gen- & Thakar, 2006). Modern pessaries erally be sterilized using an auto- are made from silicone, acrylic, clave, boiling water, or a cold ster- Shanna Atnip, MSN, WHNP-BC, is a Nurse latex, or rubber. -

Over-The-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form
Keep this catalog for future orders. Over-the-Counter (OTC) Medications and Products 2021 Beneit Catalog and Order Form Get OTC items delivered to your doorstep at no additional cost! NationsOTC.com Save Money with Your OTC Beneit Your OTC beneit allows you to purchase medications, health and wellness items, and irst aid supplies with home delivery at no additional cost. Ordering OTC Products is Simple 1 Choose Your Ordering Method Select the option that is best for you: ONLINE Visit NationsOTC.com and place an order. PHONE Call 833-SHOP-OTC (833-746-7682) TTY: 711 and speak with a Member Experience Advisor Monday-Friday, 8:00 a.m.-8:00 p.m. ET. Language support services are available, if needed. MAIL Complete the order form included in this catalog and mail to NationsOTC. 2 Select Your Products Choose products sorted by category. 3 Place Your Order Receive your delivery within 2-5 business days after your order is processed. If you have any questions or need help placing your order, we’re here for you. Member Experience Advisors are available at 833-SHOP-OTC (833-746-7682) TTY: 711 to make sure you get the items you need. 2 Keep this catalog to reference for future orders. Helpful Information Your Beneit Coverage Beneit Usage: This beneit is intended for members only. CMS does not allow this beneit to be used for your family or friends. Disenrollment: If you disenroll from your health plan, your OTC beneit will automatically end. _______________________________________________________________________________ Ordering Your OTC Products Products: Available over-the-counter items include health and wellness products that do not require a prescription. -

Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices
September 12, 2006 NOTICE Our file number: 06-120629-368 Re: Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices Health Canada is pleased to announce the release of the revised Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices. This guidance document supersedes the January 14, 2000 version of the same document. The Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices is intended to assist manufacturers in confirming the classification of medical device products after application of the Classification Rules for Medical Devices set out in Schedule 1 of the Medical Devices Regulations. This guidance document has been revised to reflect changes to the risk- based classification of particular medical device groups. Specifically, the following major changes have been made to the document: • the reclassification of specific medical device groups; • the removal of redundant information; • the addition of medical device groups; and • the omission of product groups that do not fit the meaning of a medical device as defined in the Food and Drugs Act. To search for a medical device group within the Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices PDF file, go to Edit/Find and enter a keyword, preferred name code or description in the “Find” field. For instance, to verify the risk-based classification of dental burs, enter “bur” or “drill” in the “Find” field. Please note that this guidance document is provided only as a guide and that in the event of any discrepancy between this document and the Classification Rules for Medical Devices described in the Medical Devices Regulations, the latter shall prevail. -

PEG Base S.A
Formulation Record Name: Progesterone Suppositories Strength: 200 mg Dosage Form: Suppository Route of Administration: Vaginal Date of Last Review or Revision: Today Person Completing Last Review or Revision: RPS Formula: Ingredient Quantity Physical Description Solubility Therapeutic Activity progesterone 200 mg/supp white, crystalline powder insoluble in water; soluble hormone replacement in alcohol silica gel 35.0 mg/supp white, amorphous powder insoluble in water; soluble suspending agent in hot alkaline hydroxides PEG Base s.a. Water miscible semisolid suppository base Example Calculations: Weight of suppository with only PEG Base = 2.1 gm Weight of progesterone in each suppository = 0.2 gm Density Factor of progesterone in PEG Base = 0.92 For making 10 suppositories (calculations based on 12 for anticipated loss of material): Weight of progesterone needed = 0.2 g × 12 = 2.4 g Weight of PEG needed if plain suppository = 2.1 g × 12 = 25.2 g Weight of PEG displaced: (weight of progesterone)/(density factor) = 2.4 g/0.92 = 2.6 g Weight of PEG base to weigh = 25.2 g - 2.6 g = 22.6 g Weight of Silica Gel to weigh = 35 mg × 12 = 420 mg (Note: displacement by silica gel is ignored) PEG base is 40% PEG 300 and 60% PEG 3350. Density of PEG 300 = 1.13. Weight of PEG base to weigh = 22.6 g Weight of PEG 300: 22.6 g × 0.4 = 9.0 g or 8.0 ml Weight of PEG 3350: 22.6 g × 0.6 = 13.6 g The shaded lines will need to be calculated based on the suppository weight in the individual molds.