Drug Delivery on Rectal Absorption: Suppositories
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Legal Rights of Children with Epilepsy in School and Child Care – an Advocate’S Manual
Legal Rights of Children with Epilepsy in School & Child Care AN ADVOCATE’S MANUAL AN ADVOCATE’S AN ADVOCATE’S MANUAL AN ADVOCATE’S First Edition Legal Rights of Children with Epilepsy in School and Child Care – An Advocate’s Manual First Edition Prepared by Leslie Seid Margolis Managing Attorney Maryland Disability Law Center Edited by Gary Gross Director Jeanne A. Carpenter Epilepsy Legal Defense Fund Epilepsy Foundation of America® User is hereby granted permission to copy or disseminate this publication, either in print or electronic format, provided such copies are not made, distributed or used for commercial purposes, and that the user affixes the Epilepsy Foundation’s copyright notice, and states that copying is by permission of the Epilepsy Foundation. To disseminate otherwise, or to republish, requires written permission from the Epilepsy Foundation. Permission can be obtained by contacting the Foundation’s Legal Department at 301-459-3700. The Epilepsy Foundation does not evaluate, promote or endorse commercial products, and nothing contained in this document is intended to be an endorsement of any particular treatment for seizures or epilepsy. © 2008 Epilepsy Foundation of America, Inc. Epilepsy Foundation® and Epilepsy Foundation of America® are registered trademarks of the Epilepsy Foundation of America, Inc. TABLE OF CONTENTS ACKNOWLEDGEMENTS …………………………………………….xiii ABOUT THE AUTHOR ...........................................................................xiv INTRODUCTION …………………………………………………………1 CHAPTER ONE What Do Attorneys -

Rectal Administration of Aid-In-Dying Medications
Rectal Administration of Aid-in-Dying Medications (NOTE: This is a medical procedure and requires a trained clinician who can evaluate the patient for this procedure, do a rectal exam to be sure that the procedure can be safely accomplished, and be responsible for potential complications. We do not recommend that this be done by families alone without significant direct clinician participation.) by Thalia DeWolf, RN, CHPN For questions and/or information, email [email protected] An Essential Warning: Aid-in-dying medications are a thick suspension of powders. They can clog a Macy Catheter. So while Macy Catheters are indeed wonderful and useful devices for end-of- life care, they should not be used for medical aid in dying. See below for proper materials. Pre-care: An intact, empty, warm, moist, well-perfused rectum assures thorough absorption of rectally administered aid-in-dying medications. Be sure your patient has good bowel care in the 72 hours before aid in dying. A daily soft BM is recommended. The best practice is to do an enema the day before or the morning of aid in dying. Within 24 hours of the procedure (or at the time of the procedure) a digital rectal exam must be performed by an experienced clinician (RN or physician) The clinician must ascertain that the lumen is patent and will accept the catheter; that the rectal vault is not filled with stool (small amounts of stool will not interfere with absorption of medications, large amounts, especially of thick, pasty stool, are likely to bind the medications and prevent absorption; that tumor has not invaded the rectum; that the rectum is warm and well-perfused). -

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

Consumer Education
CONSUMER EDUCATION Massachusetts General Laws Penalties for Possession or Possession with the Intent to Distribute • Consumers may not sell marijuana to any other individual • Marijuana is a class D controlled substance under the Massachusetts Controlled Substances Act - Mass. Gen. Laws. ch. 94C, § 31 Possession for Personal Use An adult may possess up to one ounce of marijuana; up to 5 grams of marijuana may be marijuana concentrate. Within a primary residence, an adult may possess up to 10 ounces of marijuana and any marijuana produced by marijuana plants cultivated on the premises. An adult who possesses more than one ounce of marijuana or marijuana products must secure the products with a lock. • Mass. Gen. Laws. ch. 94G, § 7 • Mass. Gen. Laws. ch. 94G § 13(b) Possession of more than one ounce of marijuana is punishable by a fine of $500 and/or imprisonment of up to 6 months. However, first offenders of the controlled substances act will be placed on probation and all official records relating to the conviction will be sealed upon successful completion of probation. Subsequent offenses may result in a fine of $2000 and/or imprisonment of up to 2 years. Individuals previously convicted of felonies under the controlled substances act who are arrested with over an ounce of marijuana may be subject to a fine of $2000 and/or up to 2 years of imprisonment. • Mass. Gen. Laws. ch. 94C, § 34 Possession with Intent to Distribute For first offenders, possessing less than 50 pounds of marijuana with the intent to manufacture, distribute, dispense or cultivate is punishable by a fine of $500-$5,000 and/or imprisonment of up to 2 years. -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Comprehensive Review on Herbal Toothpaste
Annals of R.S.C.B., ISSN:1583-6258, Vol. 25, Issue 4, 2021, Pages. 9509 - 9518 Received 05 March 2021; Accepted 01 April 2021. Comprehensive Review on Herbal Toothpaste Divya S1, Dr. J Suresh1*, Dr. S. Meenakshi 2 1. Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 2.Department of Prosthodontics, JSS Dental College, JSS Academy of Higher Education &Research, Mysuru-570015 Corresponding author Dr. J. Suresh Professor Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 Email: [email protected] Mobile No: 9480197611 ABSTRACT Herbal products for general as well as for oral health care have gained prominence around worldwide. People who aspire towards the use of herbal products often consider these products are relatively safer than products containing synthetic ingredients. Based on increased usage of herbal cosmetics we tried to make a comprehensive review on herbal toothpaste that helps to maintain a proper oral hygiene and free from periodontal disorder, reduce stain, gingivitis, calculus and caries. The present review gives basic information regarding antimicrobial potential of various herbs, formulationexcipients, that can be used in preparation of toothpaste. Key Words: Herbal toothpaste, Anti-microbial screening, Periodontal disorder, Gingivitis, calculus, Dental caries. INTRODUCTION In developing countries, the intensity of infections caused by certain pathogenic micro- organisms that may leads to mortality as well as morbidity in immune-suppressant patients [1]. Multiple abrasives, scent, green lead was used to remove the stain from teeth until mid- 19thcentury. In Medieval period rock salt, fine sand were the key ingredients used by Arabs for tooth cleaning.In the period 1950 AD, Dr. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

Mucosal Delivery Systems of Antihypertensive Drugs: a Practical Approach in General Practice Lukasz P
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018 Jun; 162(2):71-78. Mucosal delivery systems of antihypertensive drugs: A practical approach in general practice Lukasz P. Bialya,b, Cezary Wojcikc, Izabela Mlynarczuk-Bialya,b Patients who are unable to receive oral medication (p.o.) are a major problem in outpatient settings, especially in home health care systems. Mucosal administration of drugs offers an alternative to the oral route, especially when the parenteral mode cannot be used. There are three main pathways of mucosal administration: sublingual/buccal, intranasal and rectal. We discuss the possibility of mucosal delivery of antihypertensive drugs. Perindopril arginine and Amlodipine besylate are registered in the EU as orodispersible tablets for oromucosal delivery, however, they are not available in all countries. For this reason, we describe other drugs suitable for mucosal delivery: Captopril and Nitrendipine in the sublingual system and Metoprolol tartrate, Propranolol and Furosemide by the transrectal route. Based on the published data and common clinical practice we discuss the use of mucosal delivery systems of all these antihypertensive drugs with special attention to their pharmacokinetics. We illustrate this mini-review with a case report of the prolonged-term use of mucosal delivery of sublingual Captopril and Nitrendipine combined with rectal Metoprolol tartrate and Furosemide in a patient with severe hypertension unable to receive medication p.o. This is also a report on the first human use of Furosemide-containing suppositories as well as prolonged-term transmucosal administration of these four drugs, describing a practical approach leading to successful control of severe hyperten- sion with four antihypertensive drugs delivered via the mucosal route. -

WO 2010/044736 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 April 2010 (22.04.2010) WO 2010/044736 Al (51) International Patent Classification: (74) Agents: Bratt, Henrik et al; Jarnvagsgatan 10 A, S-251 A61K 9/24 (2006.01) A61P 25/34 (2006.01) 10 Helsingborg (SE). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/SE2009/05 1163 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (22) Date: International Filing CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, 13 October 2009 (13.10.2009) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, 0802 189-1 14 October 2008 (14.10.2008) SE SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant (for all designated States except US): McNeil AB [SE/SE]; Box 941, S-25 1 09 Helsingborg (SE). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors; and GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (75) Inventors/Applicants (for US only): LINDELL, Katari- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, na [SE/SE]; Skarhult 1325, S-241 93 Eslδv (SE). -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -
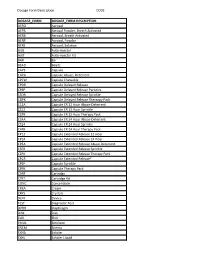
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Diazepam Rectal Gel (Diastat) Administration
LOS ANGELES UNIFIED SCHOOL DISTRICT Student Health and Human Services Division District Nursing Services DIAZEPAM RECTAL GEL (DIASTAT) ADMINISTRATION I. GENERAL GUIDELINES A. PURPOSE 1. To control acute, repetitive or prolonged seizures. 2. To prevent status epilepticus, a life-threatening condition in which seizures are continuous. 3. To administer Diastat safely and in a timely manner. B. GENERAL INFORMATION 1. Diazepam rectal gel (Diastat) is an “emergency anti-seizure medication” approved by Food and Drug Administration (FDA). Education Code 49414.7 allows unlicensed school staff to administer emergency anti-seizure medication to students with acute, prolonged or repetitive seizure 2. Licensed healthcare provider and parent authorizations for medication must be completed and signed. Healthcare Provider Authorization must specify student specific seizure symptoms, including frequency, type, duration, medication dosage, potential side effects and instructions when to call the paramedics. 3. Diastat training must be student specific- demonstration. Verbalization of the following by the trainee will occur upon completion of the training: a. Identification of characteristics of student’s seizures b. Understanding of Diastat order from the licensed healthcare provider c. Location, storage and disposal of Diastat d. Appropriate steps of administration of Diastat e. Care of the student before, during and after the administration of Diastat f. Documentation of Diastat administration 4. Parent/guardian must notify the school if Diastat was administered within the past 4 hours on a school day. C. PERSONNEL 1. School nurse or school physician 2. Designated school personnel who possess current First Aid and CPR Certification and are trained by the school nurse or the school physician.