Dosage Form FDA Data Element Number
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Rheology Measurement As a Potentially Predictive Tool for Solder Paste Transfer Efficiency and Print Volume Consistency
USING RHEOLOGY MEASUREMENT AS A POTENTIALLY PREDICTIVE TOOL FOR SOLDER PASTE TRANSFER EFFICIENCY AND PRINT VOLUME CONSISTENCY Mitch Holtzer, Karen Tellefsen and Westin Bent Alpha Assembly Solutions South Plainfield, NJ, USA [email protected] ABSTRACT Commercially available solder pastes use an upper and Industry standards such as J-STD-005 and JIS Z 3284-1994 lower limit for viscosity. Generally, this viscosity is call for the use of viscosity measurement(s) as a quality measured at one shear rate, typically the shear associated assurance test method for solder paste. Almost all solder with a spiral viscometer rotating at 10 revolutions per paste produced and sold use a viscosity range at a single minute (RPM). If the powder contained in solder paste is shear rate as part of the pass fail criteria for shipment and dissolved by the acid activator in the solder paste flux, the customer acceptance respectively. viscosity of the solder paste will quickly increase. If the viscosity increases more than 20 to 30% above the upper As had been reported many times, an estimated 80% of the limit of the specification, poor printing results will be highly defects associated with the surface mount technology likely. The solder paste will not roll evenly over the stencil process involve defects created during the printing process. and apertures in the stencil will remain unfilled, causing Viscosity at a single shear rate could predict a fatal flaw in skips in the paste printing pattern. the printability of a solder paste sample. However, false positive single shear rate viscosity readings are not The purpose of the study was to determine if there is a unknown. -

Camphor Revisited: Focus on Toxicity
Camphor Revisited: Focus on Toxicity Committee on Drugs This commentary updates a previous AAP state- TABLE. List of Camphor-Containing Products ment developed by the Committee on Drugs con- Product % of Camphor cerning camphor.1 The original commentary re- Absorbine Arthritic Pain Lotion 10 flected the level of concern among pediatric Act-On Rub Lotion 1.5 practitioners and poison centers about the toxicity of Anabaim Lotion 3 camphor. Since the original statement, the Food and Aveeno Anti-Itch Conc. Lotion 0.3 t Drug Administration (FDA) has recognized camphor Avalgesic Banaig Muscle Pain Reliever 2 as a safe and effective topical antitussive, analgesic, Bangesic t anesthetic, and antipruritic agent.2 Following the ap- Ben Gay Children’s Vaporizing Rub 5 proval process in 1983, the FDA required that the Betuline Lotion t concentration of camphor in products not exceed Campho-phemque First Aid Gel 10.8 Campho-phenique Uquid 10.85 11%.2 Fligher concentrations were not more effective Campho-phemque Powder 4.375 and could cause more serious adverse reactions if Counterpain Rub t accidentally ingested. Most reported camphor-re- Deep Down Rub 0.5 lated fatalities involved agents containing a concen- Dencorub Cream Dermal Rub t tration greater than 11%. Dermolin Liniment t Ingestion of potentially toxic substances by chil- Emul-O-Balm 1.1 dren is related to the availabffity of a product in their Heet Lotion 3 3.6 environment. Camphor remains widely available Heat Spray Minit-Rub 3.5 (Table). The toxicity of camphor when inappropri- Mollifene Ear Drops t ately used is well documented.6 Ingestion is the Musterole Regular 4 most common route of potentially toxic exposure, Panalgesic 3 with rapid onset of toxic effects. -

Absorbine Veterinary Liniment for Horses
Doc# 03.287 Ver. 11 SAFETY DATA SHEET ABSORBINE® VETERINARY LINIMENT SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION 1.1 Trade Name (as labeled): Absorbine® Veterinary Liniment Synonyms: N/A CAS No: Mixture 1.2 Product Use: Soothes sore muscles and stiff joints 1.3 Company Name: W.F. Young Company Address: 302 Benton Dr Company Address Cont: East Longmeadow, MA 01028 Business Phone: ( 413) 526-9999 Website: www.wfyoung.com 1.4 Emergency Telephone Number: (413) 526-9999 Date of Current Revision: January 17, 2017 Date of Last Revision: August 7, 2015 SECTION 2 - HAZARD IDENTIFICATION EMERGENCY OVERVIEW: This product is a green thin liquid with an acetone odor. Health Hazards: May cause skin, eye, and respiratory system irritation. Flammabilit Hazards: This product is a flammable liquid with a flash point over 20°F (-6. 7°C). Reactivit Hazards: None. Environmental Hazards: The environmental effectsof this product have not been investigated, however release may cause long term adverse environmental effects. US DOT Symbols: EU and GHS Symbols: Signal Word: Danger! 2.1 CLASSIFICATION OF SUBSTANCE OR MIXTURE IN ACCORDANCE WITH 29 CFR 1200 (OSHA HCSl AND THE EUROPEAN UNION DIRECTIVES: This product does meet the definition of a hazardous substance or preparation as defined by 29 CFR 1910. 1200 or the European Union Council Directives 67 /548/EEC, 1999/45/EC, 1272/2008/EC and subsequent Directives. EU HAZARD CLASSIFICATIONOF INGREDIENTS PER DIRECTIVE 1272/2008/EC: IndexNumber: EC# 201-939-0 This substance is not classified in the AnnexVI of Directive 67/548/EEC EC# 200-662-2 This substance is classified in the AnnexVI of Directive 67/548/EEC Index# 606-001-00-8 Substances not listed either individually or in group entries must be self classified. -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Dispensing Solder Paste with a Noflex™ Linear Drive System
AIRFREE™ WHITE PAPER LDS9000 VS. AUGER VALVE DISPENSERS Dispensing Solder Paste with a NoFlex™ Linear Drive System By: Scott Beebe, President of Fishman Corporation While the limitations of pneumatics in dispensing solder paste are well understood, higher board densities and smaller dot sizes are also challenging the performance abilities of more advanced types of dispensers, such as auger valves. AirFreeTM Linear Drive technology offers promising benefits, including significant savings in material cost and high repeatability without concern for viscosity and the volume of paste remaining in the syringe. The process of dispensing solder paste began with pneumatics. Whether a hand-held gun, or an automated workstation, whether a diaphragm, spool, needle, or piston, such dispensers depend on a column of air under pressure to force material through a dispensing tip and onto a substrate. Heat, moisture, fluctuations in air pressure, contamination, changes in viscosity... all undermine the ability of air-driven dispensers to deliver prescribed amounts of solder paste consistently, especially in the small amounts required by high density electronic circuitry (typically, less than a milligram). While pneumatic dispensers were at one time the only method available for hand-held and automatic dispensing of solder paste, the demand for precise volume control and repeatability led to the development of alternative solutions in dispensing, the most notable being the auger valve. Unfortunately, auger valves have limitations. Linear Drive technology, however, isunique, in that the amount of solder paste delivered—dot after dot after dot—is consistent, by design, and is unaffected by either changes in viscosity (Figure 1) or the amount of paste left in the syringe.* Moreover, cost savings can be substantial due to the ability to pre-package syringes with larger amounts of paste than is possible with other types of dispensers. -

Veterinary Dairy Spray Liniment by Dan Leiterman
1-888-376-6777 www.crystalcreeknatural.com December 2011 Introducing - Veterinary Dairy Spray Liniment By Dan Leiterman Crystal Creek is pleased to announce the addition of the new Veterinary Dairy Spray Liniment Features: Veterinary Dairy Spray Liniment • Available In A 24 oz. Spray Bottle Or A One Gallon (128 oz.) Refill Jug (VDSL) to our family of liniments (Veterinary Dairy Liniment and • A High Performance – Strong Relief Formula: Lini-Rub). Veterinary Dairy Spray Liniment provides the same ‘Contrast Therapy’ Warming and Cooling excellent performance as the original Analgesic Pain Relief rub-on Veterinary Dairy Liniment, only now with the convenience of a Anti-Inflammatory spray-on application. Anti-Microbial Skin Support – an aloe vera based formula Veterinary Dairy Spray Liniment is a powerful analgesic that combines • New Color For Lingering Identification Of Sprayed Animals the proven benefits of both warming and cooling ‘contrast therapy’ for • Meets National Organic Program Standards – challenged muscle tissue and edema Consider For Organic Use relief. The deep penetrating warmth soothes and supports proper • Economical To Use: circulation, while the lingering Retail Price coolness helps to reduce edema and inflammation of muscle tissue. 24 oz. Spray Bottle $29.95 / bottle If you prefer a spray-on liniment, Case Price (6 bottles/case) try Veterinary Dairy Spray Liniment and see the difference – it is stronger $28.95 / bottle and is better priced than the One Gallon Jug competition. If you prefer rub-on $134.00 / gallon liniments, try the Veterinary Dairy Case Price (4 gallons/case) Liniment (a white cream based $128.00 / gallon liniment) or the Crystal Creek Lini- Rub (an oil based liniment) and experience their excellent performance. -

Comprehensive Review on Herbal Toothpaste
Annals of R.S.C.B., ISSN:1583-6258, Vol. 25, Issue 4, 2021, Pages. 9509 - 9518 Received 05 March 2021; Accepted 01 April 2021. Comprehensive Review on Herbal Toothpaste Divya S1, Dr. J Suresh1*, Dr. S. Meenakshi 2 1. Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 2.Department of Prosthodontics, JSS Dental College, JSS Academy of Higher Education &Research, Mysuru-570015 Corresponding author Dr. J. Suresh Professor Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 Email: [email protected] Mobile No: 9480197611 ABSTRACT Herbal products for general as well as for oral health care have gained prominence around worldwide. People who aspire towards the use of herbal products often consider these products are relatively safer than products containing synthetic ingredients. Based on increased usage of herbal cosmetics we tried to make a comprehensive review on herbal toothpaste that helps to maintain a proper oral hygiene and free from periodontal disorder, reduce stain, gingivitis, calculus and caries. The present review gives basic information regarding antimicrobial potential of various herbs, formulationexcipients, that can be used in preparation of toothpaste. Key Words: Herbal toothpaste, Anti-microbial screening, Periodontal disorder, Gingivitis, calculus, Dental caries. INTRODUCTION In developing countries, the intensity of infections caused by certain pathogenic micro- organisms that may leads to mortality as well as morbidity in immune-suppressant patients [1]. Multiple abrasives, scent, green lead was used to remove the stain from teeth until mid- 19thcentury. In Medieval period rock salt, fine sand were the key ingredients used by Arabs for tooth cleaning.In the period 1950 AD, Dr. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

Making Dry Eyes Ancient History: Egypt, Mesopotamia, India, China, Greece and Rome
Making Dry Eyes Ancient History: Egypt, Mesopotamia, India, China, Greece and Rome Cheryl Lynn Bergin, OD Author’s Bio Dr. Bergin teaches Anatomy and Physiology at St. Louis Community College. She writes about systemic nutrition, ocular nutrition and the ocular effects of eating disorders. She is a member of the Optometric (Ocular) Nutrition and Optometric Historical Societies. Photo by Stan Trampe ________________________________________________________________________________ Dry eyes are not a new condition, but one might think that the case. A literature search for “dry eye” rarely reveals anything before 1970. One must be an ocular archeologist to discover how the ancients treated “irritated” eyes, dried by the harsh conditions of the desert sun and sand. EGYPT: In 1872, Georg Ebers, a German Egyptologist and novelist, discovered a collection of Egyptian medicinal recipes later called the Ebers Papyrus. The papyrus also contains ancient ophthalmic treatments. Ebers selected the important chapters on ocular diseases, translated them and added explanatory remarks. Written between 1553 and 1550 BC (1,000 years before Hippocrates), this is the oldest book on medicine (perhaps the medical textbook of ancient Egypt). It is similar to the Greek manuscripts about folk medicine that can be found among the collection of Galen and Oribasius, as well as among the manuscripts of Dioscorides. According to Ebers Papyrus, “The water within,” i.e., tear fluid and mucous secretion, is treated with incense, myrrh, and lead salt.”6,9 EGYPTIAN EYE MAKE-UP: Liz Taylor as Cleopatra16 The ancient Egyptian’s eye make-up was not just stunning, it was functional. It helped alleviate irritation from the sun’s glare off the desert sand and even prevented eye infections. -

Safety Data Sheet
First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SAFETY DATA SHEET In accordance with OSHA’s Hazard Communication Standard 29 CFR §1910.1200 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier: Absorbine® Veterinary Liniment Gel 1.2. Relevant identified uses of the substance or mixture and uses advised against Topical Analgesic 1.3. Details of the supplier of the safety data sheet Manufacturer/Supplier: W. F. Young, Inc. 302 Benton Drive East Longmeadow, MA 01028 Telephone number for information: 413 526 9999 E-mail: [email protected] 1.4. Emergency telephone number 413 526 9999 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the mixture F, Xn, Xi R10, 22, 43 2.2. Label elements Flammable Keep out of the reach of children Harmful if swallowed May product an allergic reaction 2.3. Other hazards Inhalation of vapors can cause anesthetic effect. Page 1 of 8 First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.2. Mixtures Hazardous ingredients information Component CAS Nr. EINECS / Amount DSD DSD ELINCS (%) Hazard R-Phrases Symbol Ethanola,denatured 64-17-5 200-578-6 40 - 70 F R11 Menthol 89-78-1 201-939-0 4 Choroxylenol 88-04-0 201-793-8 0.5 Xi R43 Spearmint Oil 8008-79-5 Not classified 0.5-1.0 Xi R43 Propylene Glycola 57-55-6 200-338-0 1 - 5 Other Components: Remaining components of this alcoholic mixture are proprietary, non-hazardous and/or are present at concentrations below reportable limits. -

WO 2010/044736 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 April 2010 (22.04.2010) WO 2010/044736 Al (51) International Patent Classification: (74) Agents: Bratt, Henrik et al; Jarnvagsgatan 10 A, S-251 A61K 9/24 (2006.01) A61P 25/34 (2006.01) 10 Helsingborg (SE). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/SE2009/05 1163 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (22) Date: International Filing CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, 13 October 2009 (13.10.2009) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, 0802 189-1 14 October 2008 (14.10.2008) SE SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant (for all designated States except US): McNeil AB [SE/SE]; Box 941, S-25 1 09 Helsingborg (SE). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors; and GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (75) Inventors/Applicants (for US only): LINDELL, Katari- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, na [SE/SE]; Skarhult 1325, S-241 93 Eslδv (SE). -
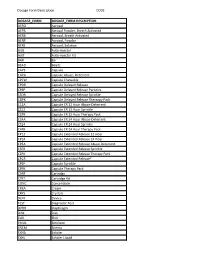
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector