We Have Some Trouble with Special Characters and Displaying Monographs. 22.09.2018 ATC Code: D04AB01 Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Camphor Revisited: Focus on Toxicity
Camphor Revisited: Focus on Toxicity Committee on Drugs This commentary updates a previous AAP state- TABLE. List of Camphor-Containing Products ment developed by the Committee on Drugs con- Product % of Camphor cerning camphor.1 The original commentary re- Absorbine Arthritic Pain Lotion 10 flected the level of concern among pediatric Act-On Rub Lotion 1.5 practitioners and poison centers about the toxicity of Anabaim Lotion 3 camphor. Since the original statement, the Food and Aveeno Anti-Itch Conc. Lotion 0.3 t Drug Administration (FDA) has recognized camphor Avalgesic Banaig Muscle Pain Reliever 2 as a safe and effective topical antitussive, analgesic, Bangesic t anesthetic, and antipruritic agent.2 Following the ap- Ben Gay Children’s Vaporizing Rub 5 proval process in 1983, the FDA required that the Betuline Lotion t concentration of camphor in products not exceed Campho-phemque First Aid Gel 10.8 Campho-phenique Uquid 10.85 11%.2 Fligher concentrations were not more effective Campho-phemque Powder 4.375 and could cause more serious adverse reactions if Counterpain Rub t accidentally ingested. Most reported camphor-re- Deep Down Rub 0.5 lated fatalities involved agents containing a concen- Dencorub Cream Dermal Rub t tration greater than 11%. Dermolin Liniment t Ingestion of potentially toxic substances by chil- Emul-O-Balm 1.1 dren is related to the availabffity of a product in their Heet Lotion 3 3.6 environment. Camphor remains widely available Heat Spray Minit-Rub 3.5 (Table). The toxicity of camphor when inappropri- Mollifene Ear Drops t ately used is well documented.6 Ingestion is the Musterole Regular 4 most common route of potentially toxic exposure, Panalgesic 3 with rapid onset of toxic effects. -

Absorbine Veterinary Liniment for Horses
Doc# 03.287 Ver. 11 SAFETY DATA SHEET ABSORBINE® VETERINARY LINIMENT SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION 1.1 Trade Name (as labeled): Absorbine® Veterinary Liniment Synonyms: N/A CAS No: Mixture 1.2 Product Use: Soothes sore muscles and stiff joints 1.3 Company Name: W.F. Young Company Address: 302 Benton Dr Company Address Cont: East Longmeadow, MA 01028 Business Phone: ( 413) 526-9999 Website: www.wfyoung.com 1.4 Emergency Telephone Number: (413) 526-9999 Date of Current Revision: January 17, 2017 Date of Last Revision: August 7, 2015 SECTION 2 - HAZARD IDENTIFICATION EMERGENCY OVERVIEW: This product is a green thin liquid with an acetone odor. Health Hazards: May cause skin, eye, and respiratory system irritation. Flammabilit Hazards: This product is a flammable liquid with a flash point over 20°F (-6. 7°C). Reactivit Hazards: None. Environmental Hazards: The environmental effectsof this product have not been investigated, however release may cause long term adverse environmental effects. US DOT Symbols: EU and GHS Symbols: Signal Word: Danger! 2.1 CLASSIFICATION OF SUBSTANCE OR MIXTURE IN ACCORDANCE WITH 29 CFR 1200 (OSHA HCSl AND THE EUROPEAN UNION DIRECTIVES: This product does meet the definition of a hazardous substance or preparation as defined by 29 CFR 1910. 1200 or the European Union Council Directives 67 /548/EEC, 1999/45/EC, 1272/2008/EC and subsequent Directives. EU HAZARD CLASSIFICATIONOF INGREDIENTS PER DIRECTIVE 1272/2008/EC: IndexNumber: EC# 201-939-0 This substance is not classified in the AnnexVI of Directive 67/548/EEC EC# 200-662-2 This substance is classified in the AnnexVI of Directive 67/548/EEC Index# 606-001-00-8 Substances not listed either individually or in group entries must be self classified. -

Veterinary Dairy Spray Liniment by Dan Leiterman
1-888-376-6777 www.crystalcreeknatural.com December 2011 Introducing - Veterinary Dairy Spray Liniment By Dan Leiterman Crystal Creek is pleased to announce the addition of the new Veterinary Dairy Spray Liniment Features: Veterinary Dairy Spray Liniment • Available In A 24 oz. Spray Bottle Or A One Gallon (128 oz.) Refill Jug (VDSL) to our family of liniments (Veterinary Dairy Liniment and • A High Performance – Strong Relief Formula: Lini-Rub). Veterinary Dairy Spray Liniment provides the same ‘Contrast Therapy’ Warming and Cooling excellent performance as the original Analgesic Pain Relief rub-on Veterinary Dairy Liniment, only now with the convenience of a Anti-Inflammatory spray-on application. Anti-Microbial Skin Support – an aloe vera based formula Veterinary Dairy Spray Liniment is a powerful analgesic that combines • New Color For Lingering Identification Of Sprayed Animals the proven benefits of both warming and cooling ‘contrast therapy’ for • Meets National Organic Program Standards – challenged muscle tissue and edema Consider For Organic Use relief. The deep penetrating warmth soothes and supports proper • Economical To Use: circulation, while the lingering Retail Price coolness helps to reduce edema and inflammation of muscle tissue. 24 oz. Spray Bottle $29.95 / bottle If you prefer a spray-on liniment, Case Price (6 bottles/case) try Veterinary Dairy Spray Liniment and see the difference – it is stronger $28.95 / bottle and is better priced than the One Gallon Jug competition. If you prefer rub-on $134.00 / gallon liniments, try the Veterinary Dairy Case Price (4 gallons/case) Liniment (a white cream based $128.00 / gallon liniment) or the Crystal Creek Lini- Rub (an oil based liniment) and experience their excellent performance. -

Safety Data Sheet
First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SAFETY DATA SHEET In accordance with OSHA’s Hazard Communication Standard 29 CFR §1910.1200 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier: Absorbine® Veterinary Liniment Gel 1.2. Relevant identified uses of the substance or mixture and uses advised against Topical Analgesic 1.3. Details of the supplier of the safety data sheet Manufacturer/Supplier: W. F. Young, Inc. 302 Benton Drive East Longmeadow, MA 01028 Telephone number for information: 413 526 9999 E-mail: [email protected] 1.4. Emergency telephone number 413 526 9999 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the mixture F, Xn, Xi R10, 22, 43 2.2. Label elements Flammable Keep out of the reach of children Harmful if swallowed May product an allergic reaction 2.3. Other hazards Inhalation of vapors can cause anesthetic effect. Page 1 of 8 First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.2. Mixtures Hazardous ingredients information Component CAS Nr. EINECS / Amount DSD DSD ELINCS (%) Hazard R-Phrases Symbol Ethanola,denatured 64-17-5 200-578-6 40 - 70 F R11 Menthol 89-78-1 201-939-0 4 Choroxylenol 88-04-0 201-793-8 0.5 Xi R43 Spearmint Oil 8008-79-5 Not classified 0.5-1.0 Xi R43 Propylene Glycola 57-55-6 200-338-0 1 - 5 Other Components: Remaining components of this alcoholic mixture are proprietary, non-hazardous and/or are present at concentrations below reportable limits. -

Dosage Form FDA Data Element Number
Home Drugs Development & Approval Process (Drugs) Forms & Submission Requirements Electronic Submissions to CDER Data Standards Manual (monographs) Drugs Dosage Form FDA Data Element Number. None. CDER Data Element Number. C-DRG-00201 Data Element Name. Dosage Form. Data Element OID: 2.16.840.1.113883.3.26.1.1.2 Data Element NCI Concept ID: C42636 Version Number. 008 Description. This standard provides for all drug dosage forms. The granularity of data often requires that more specific dosage form terms be stored in automated databases than are represented in publications. These dosage form terms are available not only for use in databases that track approved drug products, but also for drug products such as: those that have not been approved, investigational drug products, homeopathic drug products, biologic products, veterinary drug products, and bulk drug products. A ‘use restrictions’ column is being added to help identify where a particular dosage form set or subset should be used (a=CDER Databases, b=NDC Directory, c=Orange Book). The definitions for Lotion, Cream, Ointment, and Paste were revised on June 21, 2006 to include information that would assist the user in differentiating between these dosage forms. These changes were the result of discussions during an FDA Advisory Committee with an open public forum, scientific studies that were published in refereed pharmaceutical journals, and internal discussions by Agency personnel, including the usual adherence to the criteria that are specified in MaPP 7600.4. A 1999 Food and Drug Administration Draft Guidance for Industry states: "A dosage form is the way of identifying the drug in its physical form. -

Hinkson Creek Comprehensive Chemical Analysis Proposal
UNITED STATES GOVERNMENT memorandum DATE: August 29, 2019 REPLY TO ATTN OF: David Alvarez, USGS, 573-441-2970, [email protected] SUBJECT: Cost estimate for the analysis of water and sediment samples from Hinkson Creek TO: Lynne Hooper, Boone County Resource Management, [email protected] Investigation of continued causes of impairment in Hinkson Creek is of interest to the Hinkson Creek Science Team. Some work has been done looking at basic water quality parameters, but little data exists looking at organic and inorganic contaminants which may be related to increased urbanization in the watershed. The Environmental Chemistry Branch was asked to develop a sampling plan which includes potential indicator chemicals that may indicate an increased contaminant loading into the Creek. Below is an estimate for the chemical analysis of water and sediment samples from Hinkson Creek. The costs below represent totals for the sampling at 5 sites each during an upcoming Fall and Spring season. Options for both water and sediment analyzes are included. Proposed chemicals to be investigated include: a suite of metals typical of urban environments, current use pesticides (CUP) related to agriculture, wastewater indicators (WI), polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides, polychlorinated biphenyls (total PCBs), and polybrominated diphenyl ether (PBDE) flame retardants. A tentative list of analytes is provided as an attachment to this memo. In addition to the specific chemical analyses, a screen for total estrogenicity of chemicals will be run using the in vitro yeast estrogen screen (YES). The YES assay is a cell-based assay where estrogens or estrogen-mimicking chemicals bind to an estrogen receptor which can be measured. -

Pharmaceutical Compounding and Dispensing Sample Chapter
Copyright Pharmaceutical Press www.pharmpress.com 6 Solutions Introduction and overview 101 Gargles and mouthwashes 105 General principles of solution preparation 103 Enemas and douches 105 Solubility 103 External solutions 105 Stability 103 Lotions 105 General method 103 Liniments 105 Oral solutions 104 Applications 105 Elixirs 104 Collodions 106 Linctuses 105 Worked examples 106 Syrups 105 Summary of essential principles relating to solutions 112 Mixtures 105 Packaging 112 Draughts 105 Discard dates 112 Spirits 105 Labelling 113 Paediatric drops 105 Introduction and overview Essentially a solution is a homogeneous liquid preparation that contains one or more dissolved med- Solutions are one of the oldest dosage forms used in the icaments. Since, by definition, active ingredients are treatment of patients and afford rapid and high dissolved within the vehicle, uniform doses by volume absorption of soluble medicinal products. Therefore, may be obtained without any need to shake the for- the compounding of solutions retains an important mulation. This is an advantage over some other for- place in therapeutics today. Owing to the simplicity mulation types (e.g. suspensions, see Chapter 7). and hence the speed of preparation of an ad hoc for- In general, water is chosen as the vehicle in which mulation, they are of particular use for individuals medicaments are dissolved, as it is non-toxic, non- who have difficulty in swallowing solid dosage forms irritant, tasteless, relatively cheap, and many drugs (for example paediatric, geriatric, intensive care and are water soluble. Problems may be encountered psychiatric patients), where compliance needs to be where active drugs are not particularly water soluble checked on administration (for example in prisons or or suffer from hydrolysis in aqueous solution. -

Pressurized Liquid Extraction Using Water/Isopropanol Coupled with Solid-Phase Extraction Cleanup for Industrial and Anthropogen
Analytica Chimica Acta 534 (2005) 89–100 Pressurized liquid extraction using water/isopropanol coupled with solid-phase extraction cleanup for industrial and anthropogenic waste-indicator compounds in sediment Mark R. Burkhardt∗, Rhiannon C. ReVello, Steven G. Smith, Steven D. Zaugg U.S. Geological Survey, Box 25046, MS 407, Denver, CO 80225-0046, USA Received 15 October 2004; received in revised form 8 November 2004; accepted 8 November 2004 Available online 21 December 2004 Abstract A broad range of organic compounds is recognized as environmentally relevant for their potential adverse effects on human and ecosystem health. This method was developed to better determine the distribution of 61 compounds that are typically associated with industrial and household waste as well as some that are toxic and known (or suspected) for endocrine-disrupting potential extracted from environmental sediment samples. Pressurized liquid extraction (PLE) coupled with solid-phase extraction (SPE) was used to reduce sample preparation time, reduce solvent consumption to one-fifth of that required using dichloromethane-based Soxhlet extraction, and to minimize background interferences for full scan GC/MS analysis. Recoveries from spiked Ottawa sand, commercially available topsoil, and environmental stream sediment, fortified at 4–720 g per compound, averaged 76 ± 13%. Initial method detection limits for single-component compounds ranged from 12.5 to 520 g/kg, based on 25 g samples. Results from 103 environmental sediment samples show that 36 out of 61 compounds (59%) were detected in at least one sample with concentrations ranging from 20 to 100,000 g/kg. The most frequently detected compound, beta- sitosterol, a plant sterol, was detected in 87 of the 103 (84.5%) environmental samples with a concentration range 360–100,000 g/kg. -

Queensland Health List of Approved Medicines
Queensland Health List of Approved Medicines Drug Form Strength Restriction abacavir * For use in accord with PBS Section 100 indications * oral liquid See above 20 mg/mL See above tablet See above 300 mg See above abacavir + lamivudine * For use in accord with PBS Section 100 indications * tablet See above 600 mg + 300 mg See above abacavir + lamivudine + * For use in accord with PBS Section 100 indications * zidovudine tablet See above 300 mg + 150 mg + 300 mg See above abatacept injection 250 mg * For use in accord with PBS Section 100 indications * abciximab (a) Interventional Cardiologists for complex angioplasty (b) Interventional and Neuro-interventional Radiologists for rescue treatment of thromboembolic events that occur during neuroendovascular procedures. * Where a medicine is not TGA approved, patients should be made fully aware of the status of the medicine and appropriate consent obtained * injection See above 10 mg/5 mL See above abiraterone For use by medical oncologists as per the PBS indications for outpatient and discharge use only tablet See above 250 mg See above 500 mg See above acamprosate Drug and alcohol treatment physicians for use with a comprehensive treatment program for alcohol dependence with the goal of maintaining abstinence. enteric tablet See above 333 mg See above acarbose For non-insulin dependent diabetics with inadequate control despite diet; exercise and maximal tolerated doses of other anti-diabetic agents tablet See above 50 mg See above 100 mg See above acetazolamide injection 500 mg tablet 250 mg acetic acid ear drops 3% 15mL solution 2% 100mL green 3% 1 litre 6% 1 Litre 6% 200mL Generated on: 30-Aug-2021 Page 1 of 142 Drug Form Strength Restriction acetylcysteine injection For management of paracetamol overdose 2 g/10 mL See above 6 g/30 mL See above aciclovir cream Infectious disease physicians, haematologists and oncologists 5% See above eye ointment For use on the advice of Ophthalmologists only. -
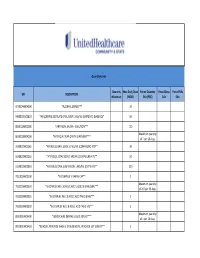
UHC PA Community and State Quantity Limit List
Quantity Limits Quantity Max Daily Dose Period Quantity Period Days Period Fills GPI DESCRIPTION Maximum (MDD) Edit (PQE) Edit Edit 97703040004300 *ALCOHOL SWABS*** 10 34000003101810 *AMLODIPINE BESYLATE ORAL SUSP 1 MG/ML (CMPD KIT) (BASE EQ)* 10 88501000002000 *ARTIFICIAL SALIVA - SOLUTION*** 120 Maximum quantity 86202000004200 *ARTIFICIAL TEAR OPHTH OINTMENT*** of 7 per 26 days. 33200020002065 *ATENOLOL ORAL SOLN 10 MG/ML (COMPOUND KIT)** 20 33200020002055 *ATENOLOL ORAL SOLN 2 MG/ML (COMPOUND KIT)** 50 33200020001810 *ATENOLOL ORAL SUSPENSION 1 MG/ML (CMPD KIT)** 200 78110000000100 *B-COMPLEX VITAMIN CAP** 1 Maximum quantity 78133000000920 *B-COMPLEX W/ C & FOLIC ACID LIQUID 0.9 MG/5ML*** of 237 per 26 days. 78133000000325 *B-COMPLEX W/ C & FOLIC ACID TAB 0.8 MG*** 1 78133000000330 *B-COMPLEX W/ C & FOLIC ACID TAB 1 MG*** 1 Maximum quantity 88350010006400 *BENZOCAINE DENTAL LIQUID 20% KIT*** of 1 per 26 days. 90050010006410 *BENZOYL PEROXIDE WASH 2.5% & BENZOYL PEROXIDE LOT 10% KIT** 1 47300005100100 *BIFIDOBACTERIUM BIFIDUM CAP** 2 97202007100900 *BLOOD GLUCOSE CALIBRATION - LIQUID*** 0.04 Maximum quantity 97202011006200 *BLOOD GLUCOSE METER DISPOSABLE DEVICE WITH TEST STRIPS*** of 1 per 999 days. Maximum quantity 97202010006200 *BLOOD GLUCOSE MONITORING DEVICES*** of 1 per 365 days. Maximum quantity 97202010006410 *BLOOD GLUCOSE MONITORING KIT W/ DEVICE*** of 1 per 365 days. Maximum quantity 97202010006400 *BLOOD GLUCOSE MONITORING KIT*** of 1 per 365 days. Maximum quantity 97750010006200 *BLOOD PRESSURE MONITORING - DEVICE*** of 1 per 999 days. Maximum quantity 9025990212B120 *CALCIPOTRIENE CR 0.005% & DIMETHICONE CR 5% THERAPY PACK*** of 1 per 26 days. 79109903450340 *CALCIUM CARB-VIT D W/ MINERALS TABS 600 MG-200 UNIT*** 5 79109903450355 *CALCIUM CARB-VIT D W/ MINERALS TABS 600 MG-800 UNIT*** 2 Maximum quantity 6610990420B120 *CELECOXIB CAP 200 MG & METH SAL-MEN-CAPSAICIN LIQD THER PK* of 1 per 26 days. -

Cosmedix Active Ingredients Glossary
COSMEDIX ACTIVE INGREDIENTS GLOSSARY INCI Name Function Acetyl Hexapeptide-1 Is a biomimetic peptide antagonist specific of the alpha-melanocyte stimulating hormone by preventing any further activation of the tyrosinase, and thus blocking melanin synthesis. Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate (ATP) and protein synthesis, as a chemical component of DNA and . Alcohol Carrier, Solubilizer and Antiseptic Alcohol Denat. Carrier, Solubilizer and Antiseptic Allantoin it is derived from the extracts of a comfrey plant. It softens the skin and enables it to absorb more moisture. It’s particularly effective at treating wounds, burns, skin ulcers, eczema, and any other abrasion in the skin. Aloe Barbadensis Leaf Juice Powder it is a species of succulent plant in the genus Aloe that grows in arid climates and is widely distributed in Africa, India, and other arid areas. As a soothing, moisturizing and conditioning agent, Aloe vera extracts may be useful in the treatment of wound and burn healing, minor skin infections, Sebaceous cyst, diabetes, and elevated blood lipids in humans.These positive effects are thought to be due to the presence of compounds such as polysaccharides, mannans, anthraquinones, and lectins. Amino Esters-1 Skin-Conditioning Agent - Aminoguanidine HCL It is an investigational drug for the treatment of diabetic nephropathy. It is a diamine oxidase and nitric oxide synthase inhibitor and acts as an anti-oxidant that helps reducing the formation of advanced glycation end-products (AGEs) which destroy collagen and contribute skin aging. Arabinogalactan Protein (AGP) is a polysaccharide extracted from larch trees. -

Table 10. Chemicals Analyzed in the Wastewater Compound Schedule (Zaugg and Others, 2002). [USGS, U.S
Table 10. Chemicals analyzed in the wastewater compound schedule (Zaugg and others, 2002). [USGS, U.S. Geological Survey] Laboratory USGS Chemical Abstract reporting level Chemical Use or source parameter Service (CAS) (micrograms per code Registry Number1 liter) Acetophenone Fragrance/flavor 62064 98-86-2 0.1 Acetyl hexamethyl tetrahydronaphthalene (AHTN) Fragrance/flavor 62065 21145-77-7 0.5 Anthracene Wood preservative, component of tar, diesel or crude oil 34221 120-12-7 0.08 9,10-Anthraquinone Manufacturing dye and textiles, seed treatment, bird repellent 62066 84-65-1 0.16 Benzo[a]pyrene Hydrocarbon, used in cancer research, combustion product 34248 50-32-8 0.12 Benzophenone Fixative for perfumes and soap 62067 119-61-9 0.18 Bisphenol A Manufacturing polycarbonate resins, antioxidant, flame retardant 62069 80-05-7 0.4 Bromacil General use herbicide 4029 314-40-9 0.4 Bromoform Wastewater ozination byproduct, military/explosives 34288 75-25-2 0.08 3-tert -Butyl-4-hydroxy anisole (BHA) Antioxidant, general preservative 62059 25013-16-5 0.6 Caffeine Beverages, diuretic, very mobile and biodegradable 50305 58-08-2 0.2 Camphor Flavor, odorant, ointments 62070 76-22-2 0.1 Carbaryl Insecticide, crop and garden uses 82680 63-25-2 1 Carbazole Insecticide, manufacturing dyes, explosives, and lubricants 62071 86-74-8 0.08 Chlorpyrifos Insecticide, domestic pest and termite control 38933 2921-88-2 0.2 Cholesterol Often a fecal indicator, also a plant sterol 62072 57-88-5 1.4 3-beta -Coprostanol Carnivore fecal indicator 62057 360-68-9 1.6 Cotinine