UHC PA Community and State Quantity Limit List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Camphor Revisited: Focus on Toxicity
Camphor Revisited: Focus on Toxicity Committee on Drugs This commentary updates a previous AAP state- TABLE. List of Camphor-Containing Products ment developed by the Committee on Drugs con- Product % of Camphor cerning camphor.1 The original commentary re- Absorbine Arthritic Pain Lotion 10 flected the level of concern among pediatric Act-On Rub Lotion 1.5 practitioners and poison centers about the toxicity of Anabaim Lotion 3 camphor. Since the original statement, the Food and Aveeno Anti-Itch Conc. Lotion 0.3 t Drug Administration (FDA) has recognized camphor Avalgesic Banaig Muscle Pain Reliever 2 as a safe and effective topical antitussive, analgesic, Bangesic t anesthetic, and antipruritic agent.2 Following the ap- Ben Gay Children’s Vaporizing Rub 5 proval process in 1983, the FDA required that the Betuline Lotion t concentration of camphor in products not exceed Campho-phemque First Aid Gel 10.8 Campho-phenique Uquid 10.85 11%.2 Fligher concentrations were not more effective Campho-phemque Powder 4.375 and could cause more serious adverse reactions if Counterpain Rub t accidentally ingested. Most reported camphor-re- Deep Down Rub 0.5 lated fatalities involved agents containing a concen- Dencorub Cream Dermal Rub t tration greater than 11%. Dermolin Liniment t Ingestion of potentially toxic substances by chil- Emul-O-Balm 1.1 dren is related to the availabffity of a product in their Heet Lotion 3 3.6 environment. Camphor remains widely available Heat Spray Minit-Rub 3.5 (Table). The toxicity of camphor when inappropri- Mollifene Ear Drops t ately used is well documented.6 Ingestion is the Musterole Regular 4 most common route of potentially toxic exposure, Panalgesic 3 with rapid onset of toxic effects. -

Absorbine Veterinary Liniment for Horses
Doc# 03.287 Ver. 11 SAFETY DATA SHEET ABSORBINE® VETERINARY LINIMENT SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION 1.1 Trade Name (as labeled): Absorbine® Veterinary Liniment Synonyms: N/A CAS No: Mixture 1.2 Product Use: Soothes sore muscles and stiff joints 1.3 Company Name: W.F. Young Company Address: 302 Benton Dr Company Address Cont: East Longmeadow, MA 01028 Business Phone: ( 413) 526-9999 Website: www.wfyoung.com 1.4 Emergency Telephone Number: (413) 526-9999 Date of Current Revision: January 17, 2017 Date of Last Revision: August 7, 2015 SECTION 2 - HAZARD IDENTIFICATION EMERGENCY OVERVIEW: This product is a green thin liquid with an acetone odor. Health Hazards: May cause skin, eye, and respiratory system irritation. Flammabilit Hazards: This product is a flammable liquid with a flash point over 20°F (-6. 7°C). Reactivit Hazards: None. Environmental Hazards: The environmental effectsof this product have not been investigated, however release may cause long term adverse environmental effects. US DOT Symbols: EU and GHS Symbols: Signal Word: Danger! 2.1 CLASSIFICATION OF SUBSTANCE OR MIXTURE IN ACCORDANCE WITH 29 CFR 1200 (OSHA HCSl AND THE EUROPEAN UNION DIRECTIVES: This product does meet the definition of a hazardous substance or preparation as defined by 29 CFR 1910. 1200 or the European Union Council Directives 67 /548/EEC, 1999/45/EC, 1272/2008/EC and subsequent Directives. EU HAZARD CLASSIFICATIONOF INGREDIENTS PER DIRECTIVE 1272/2008/EC: IndexNumber: EC# 201-939-0 This substance is not classified in the AnnexVI of Directive 67/548/EEC EC# 200-662-2 This substance is classified in the AnnexVI of Directive 67/548/EEC Index# 606-001-00-8 Substances not listed either individually or in group entries must be self classified. -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Veterinary Dairy Spray Liniment by Dan Leiterman
1-888-376-6777 www.crystalcreeknatural.com December 2011 Introducing - Veterinary Dairy Spray Liniment By Dan Leiterman Crystal Creek is pleased to announce the addition of the new Veterinary Dairy Spray Liniment Features: Veterinary Dairy Spray Liniment • Available In A 24 oz. Spray Bottle Or A One Gallon (128 oz.) Refill Jug (VDSL) to our family of liniments (Veterinary Dairy Liniment and • A High Performance – Strong Relief Formula: Lini-Rub). Veterinary Dairy Spray Liniment provides the same ‘Contrast Therapy’ Warming and Cooling excellent performance as the original Analgesic Pain Relief rub-on Veterinary Dairy Liniment, only now with the convenience of a Anti-Inflammatory spray-on application. Anti-Microbial Skin Support – an aloe vera based formula Veterinary Dairy Spray Liniment is a powerful analgesic that combines • New Color For Lingering Identification Of Sprayed Animals the proven benefits of both warming and cooling ‘contrast therapy’ for • Meets National Organic Program Standards – challenged muscle tissue and edema Consider For Organic Use relief. The deep penetrating warmth soothes and supports proper • Economical To Use: circulation, while the lingering Retail Price coolness helps to reduce edema and inflammation of muscle tissue. 24 oz. Spray Bottle $29.95 / bottle If you prefer a spray-on liniment, Case Price (6 bottles/case) try Veterinary Dairy Spray Liniment and see the difference – it is stronger $28.95 / bottle and is better priced than the One Gallon Jug competition. If you prefer rub-on $134.00 / gallon liniments, try the Veterinary Dairy Case Price (4 gallons/case) Liniment (a white cream based $128.00 / gallon liniment) or the Crystal Creek Lini- Rub (an oil based liniment) and experience their excellent performance. -

ANNOVERA™ (Segesterone Acetate and Ethinyl Estradiol Vaginal System) • Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ANNOVERA™ no earlier than 4 weeks after delivery, in females who These highlights do not include all the information needed to use are not breastfeeding. Consider cardiovascular risk factors before ANNOVERA™ safely and effectively. initiating in all females, particularly those over 35 years. (5.1, 5.5) See Full Prescribing Information for ANNOVERA™. • Liver Disease: Discontinue if jaundice occurs. (5.2) ANNOVERA™ (segesterone acetate and ethinyl estradiol vaginal system) • Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Initial U.S. Approval: 2018 Treatment: Stop ANNOVERA™ prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir. ANNOVERA™ can be restarted 2 weeks following completion of this WARNING: CIGARETTE SMOKING AND regimen. (5.3) SERIOUS CARDIOVASCULAR EVENTS • Hypertension: Do not prescribe ANNOVERA™ for females with See full prescribing information for complete boxed warning. uncontrolled hypertension or hypertension with vascular disease. If • Females over 35 years old who smoke should not use used in females with well-controlled hypertension, monitor blood ANNOVERA™. (4) pressure and stop use if blood pressure rises significantly. (5.4) • Cigarette smoking increases the risk of serious cardiovascular • Carbohydrate and lipid metabolic effects: Monitor glucose in pre events from combination hormonal contraceptive (CHC) use. (4) diabetic and diabetic females taking ANNOVERA™. Consider an alternate contraceptive method for females with uncontrolled ----------------------------INDICATIONS AND USAGE-------------------------- dyslipidemias. (5.7) ANNOVERA™ is a progestin/estrogen CHC indicated for use by females of • Headache: Evaluate significant change in headaches and discontinue reproductive potential to prevent pregnancy. (1) ANNOVERA™ if indicated. (5.8) Limitation of use: Not adequately evaluated in females with a body mass index • Bleeding Irregularities and Amenorrhea: May cause irregular bleeding of >29 kg/m2. -

209627Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209627Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review Reviewers of Multi-Disciplinary Review and Evaluation SECTIONS OFFICE/ AUTHORED/ ACKNOWLEDGED/ DISCIPLINE REVIEWER DIVISION APPROVED Mark Seggel, Ph.D. OPQ/ONDP/DNDP2 Authored: Section 4.2 Digitally signed by Mark R. Seggel -S CMC Lead DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, cn=Mark R. Signature: Mark R. Seggel -S Seggel -S, 0.9.2342.19200300.100.1.1=1300071539 Date: 2018.08.08 16:29:15 -04'00' Frederic Moulin, DVM, PhD OND/ODE3/DBRUP Authored: Section 5 Pharmacology/ Digitally signed by Frederic Moulin -S Toxicology DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Reviewer Signature: Frederic Moulin -S 0.9.2342.19200300.100.1.1=2001708658, cn=Frederic Moulin -S Date: 2018.08.08 15:26:57 -04'00' Kimberly Hatfield, PhD OND/ODE3/DBRUP Approved: Section 5 Pharmacology/ Toxicology Digitally signed by Kimberly P. Hatfield -S DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Team Leader Signature: Kimberly P. Hatfield -S 0.9.2342.19200300.100.1.1=1300387215, cn=Kimberly P. Hatfield -S Date: 2018.08.08 14:56:10 -04'00' Li Li, Ph.D. OCP/DCP3 Authored: Sections 6 and 17.3 Clinical Pharmacology Dig ta ly signed by Li Li S DN c=US o=U S Government ou=HHS ou=FDA ou=People Reviewer cn=Li Li S Signature: Li Li -S 0 9 2342 19200300 100 1 1=20005 08577 Date 2018 08 08 15 39 23 04'00' Doanh Tran, Ph.D. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

Health Care Reform
Health Care Reform Preventative Drug list At Magellan Rx Management, we are driven to helping our clients most effectively manage changes in the prescription drug environment. As part of the Patient Protection and Affordable Care Act (PPACA), effective on or after August 1, 2012 non-grandfathered plans are required to cover select FDA-approved drug products related to preventive health services for Adults, Children and Women without a member having to pay a copayment, co-insurance, or meet a deductible. As your pharmacy benefits manager, we will support coverage of specific products at $0 copay as prescription benefit plans become subject to the law. Magellan Rx Management will also routinely update FDA-approved product lists to comply with the Preventive Health mandates and require prescriptions for product coverage. MRx has created ten specific lists that address the preventive health requirements above. A plan is required to cover all the drug lists below, either through the pharmacy or medical benefits. If the plan needs advice regarding coverage, MRx recommends the plan connect with their respective legal counsel. The content of the list will be maintained by Magellan Rx Management and will be continually reviewed and updated to ensure compliance with healthcare reform mandates. UPDATE: For plan years beginning on or after September 24, 2014 (January 1, 2015 for calendar year plans), non-grandfathered health plans are required to cover prescription medications designed to reduce the risk of breast cancer in women, without cost-sharing, subject to reasonable medical management. These required drugs have been added to the “Preventive Health for Adults, Children and Women: Prescription and OTC Products” list below. -

Vaginal Administration of Contraceptives
Scientia Pharmaceutica Review Vaginal Administration of Contraceptives Esmat Jalalvandi 1,*, Hafez Jafari 2 , Christiani A. Amorim 3 , Denise Freitas Siqueira Petri 4 , Lei Nie 5,* and Amin Shavandi 2,* 1 School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, UK 2 BioMatter Unit, École Polytechnique de Bruxelles, Université Libre de Bruxelles, Avenue F.D. Roosevelt, 50-CP 165/61, 1050 Brussels, Belgium; [email protected] 3 Pôle de Recherche en Gynécologie, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, 1200 Brussels, Belgium; [email protected] 4 Fundamental Chemistry Department, Institute of Chemistry, University of São Paulo, Av. Prof. Lineu Prestes 748, São Paulo 05508-000, Brazil; [email protected] 5 College of Life Sciences, Xinyang Normal University, Xinyang 464000, China * Correspondence: [email protected] (E.J.); [email protected] (L.N.); [email protected] (A.S.); Tel.: +32-2-650-3681 (A.S.) Abstract: While contraceptive drugs have enabled many people to decide when they want to have a baby, more than 100 million unintended pregnancies each year in the world may indicate the contraceptive requirement of many people has not been well addressed yet. The vagina is a well- established and practical route for the delivery of various pharmacological molecules, including contraceptives. This review aims to present an overview of different contraceptive methods focusing on the vaginal route of delivery for contraceptives, including current developments, discussing the potentials and limitations of the modern methods, designs, and how well each method performs for delivering the contraceptives and preventing pregnancy. -

Safety Data Sheet
First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SAFETY DATA SHEET In accordance with OSHA’s Hazard Communication Standard 29 CFR §1910.1200 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier: Absorbine® Veterinary Liniment Gel 1.2. Relevant identified uses of the substance or mixture and uses advised against Topical Analgesic 1.3. Details of the supplier of the safety data sheet Manufacturer/Supplier: W. F. Young, Inc. 302 Benton Drive East Longmeadow, MA 01028 Telephone number for information: 413 526 9999 E-mail: [email protected] 1.4. Emergency telephone number 413 526 9999 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the mixture F, Xn, Xi R10, 22, 43 2.2. Label elements Flammable Keep out of the reach of children Harmful if swallowed May product an allergic reaction 2.3. Other hazards Inhalation of vapors can cause anesthetic effect. Page 1 of 8 First issue: September 17, 2009 Revision date: October 27, 2014 SDS No. 007.636 Version: 5 SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.2. Mixtures Hazardous ingredients information Component CAS Nr. EINECS / Amount DSD DSD ELINCS (%) Hazard R-Phrases Symbol Ethanola,denatured 64-17-5 200-578-6 40 - 70 F R11 Menthol 89-78-1 201-939-0 4 Choroxylenol 88-04-0 201-793-8 0.5 Xi R43 Spearmint Oil 8008-79-5 Not classified 0.5-1.0 Xi R43 Propylene Glycola 57-55-6 200-338-0 1 - 5 Other Components: Remaining components of this alcoholic mixture are proprietary, non-hazardous and/or are present at concentrations below reportable limits. -
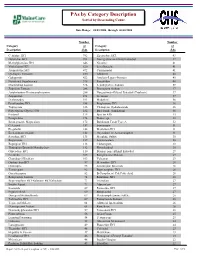
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Oral Contraceptives
ORAL CONTRACEPTIVES STRENGTH DRUG ESTROGEN PROGESTIN (ESTROGEN/PROGESTIN) MONOPHASIC Aviane 28, Lessina, Lutera, Orsythia, Ethinyl estradiol Levonorgestrel 20mcg/0.1mg Sronyx† Beyaz*, YAZ Ethinyl estradiol Drospirenone 20mcg/3mg [Gianvi, Loryna, Vestura]† Brevicon, Modicon Ethinyl estradiol Norethindrone 35mcg/0.5mg [Necon 0.5/35, Nortrel 0.5/35]† Desogen Ethinyl estradiol Desogestrel 30mcg/0.15mg [Apri, Emoquette, Reclipsen, Solia]† Femcon Fe, Ovcon 35 Ethinyl estradiol Norethindrone 35mcg/0.4mg [Balziva, Briellyn, Philith, Vyfemla Zenchent, Zenchent Fe]† Loestrin 21 1/20, Loestrin Fe 1/20, Ethinyl estradiol Norethindrone acetate 20mcg/1mg Loestrin 24 Fe, Minastrin 24 Fe [Gildess Fe 1/20, Junel 1/20, Junel Fe 1/20, Larin 1/20, Larin Fe 1/20, Microgestin 1/20, Microgestin Fe 1/20]† Loestrin 21 1.5/30, Loestrin Fe 1.5/30 Ethinyl estradiol Norethindrone acetate 30mcg/1.5mg [Gildess Fe 1.5/30, Junel 1.5/30, Junel Fe 1.5/30, Microgestin 1.5/30, Microgestin Fe 1.5/30]† Lo/Ovral Ethinyl estradiol Norgestrel 30mcg/0.3mg [Cryselle, Low-Ogestrel]† Lybrel Ethinyl estradiol Levonorgestrel 20mcg/0.09mg [Amethyst]† [Altavera, Levora, Marlissa, Portia]† Ethinyl estradiol Levonorgestrel 30mcg/0.15mg Norinyl 1/35, Ortho-Novum 1/35 Ethinyl estradiol Norethindrone 35mcg/1mg [Alyacen 1/35, Cyclafem 1/35, Dasetta 1/35, Necon 1/35, Nortrel 1/35]† Necon 1/50, Norinyl 1/50 Mestranol Norethindrone 50mcg/1mg Ogestrel 0.5/50† Ethinyl estradiol Norgestrel 50mcg/0.5mg Ortho-Cyclen Ethinyl estradiol Norgestimate 35mcg/0.25mg [MonoNessa, Previfem, Sprintec]† Ovcon