Order in Council 1243/1995
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening/Spot Test of Narcotics
Indian Journal of Forensic and Community Medicine 2020;7(4):160–165 Content available at: https://www.ipinnovative.com/open-access-journals Indian Journal of Forensic and Community Medicine Journal homepage: https://www.ipinnovative.com/journals/IJFCM Review Article Screening/spot test of narcotics A K Jaiswal1,*, Kamna Sharma2, Rohit Kanojia3, Sally Lukose4 1Dept. of Forensic Medicine & Toxicology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 2Galgotias University, Greater Noida, Uttar Pradesh, India 3Dept. of Chemistry, University of Delhi, New Delhi, India 4CTM-IRTE, Faridabad, Haryana, India ARTICLEINFO ABSTRACT Article history: Narcotics are the substances used to treat moderate to severe pain. They could be natural like opiates such Received 25-11-2020 as morphine, codeine etc., synthetic like fentanyl, methadone etc., and semi-synthetic like oxycodone, Accepted 02-12-2020 hydrocodone etc. These drugs act as pain relievers, induces the state of stupor or sleep, and increase Available online 08-01-2021 the physical dependence on them. In forensic autopsy case, the forensic pathologist may require a complete toxicological investigation for different poisons including stimulants. In India, Forensic Science Laboratories run by Government under the Home ministry usually carry out this. The samples must be Keywords: analysed by the forensic toxicologist/chemists/scientist. This article deals with the screening/spot test for Narcotics narcotics. It attempts to simplify the standard procedures in a step-wise manner, which can be of handy Screening reference for the forensic toxicologist. Spot test Drugs © This is an open access article distributed under the terms of the Creative Commons Attribution Opioids etc License (https://creativecommons.org/licenses/by/4.0/) which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Medicines Regulations 1984 (SR 1984/143)
Reprint as at 1 July 2014 Medicines Regulations 1984 (SR 1984/143) David Beattie, Governor-General Order in Council At the Government House at Wellington this 5th day of June 1984 Present: His Excellency the Governor-General in Council Pursuant to section 105 of the Medicines Act 1981, and, in the case of Part 3 of the regulations, to section 62 of that Act, His Excellency the Governor-General, acting on the advice of the Minister of Health tendered after consultation with the organisations and bodies that ap- peared to the Minister to be representatives of persons likely to be substantially affected, and by and with the advice and consent of the Executive Council, hereby makes the following regulations. Contents Page 1 Title and commencement 5 Note Changes authorised by subpart 2 of Part 2 of the Legislation Act 2012 have been made in this official reprint. Note 4 at the end of this reprint provides a list of the amendments incorporated. These regulations are administered by the Ministry of Health. 1 Reprinted as at Medicines Regulations 1984 1 July 2014 2 Interpretation 5 Part 1 Classification of medicines 3 Classification of medicines 11 Part 2 Standards 4 Standards for medicines, related products, medical 11 devices, cosmetics, and surgical dressings 5 Pharmacist may dilute medicine in particular case 12 6 Colouring substances [Revoked] 12 Part 3 Advertisements 7 Advertisements not to claim official approval 13 8 Advertisements for medicines 13 9 Advertisements for related products 15 10 Advertisements for medical devices 15 11 Advertisements -

Medical Management of Biologic Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Fourth Edition February 2001 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES ¨ FORT DETRICK FREDERICK, MARYLAND 1 Sources of information: National Response Center 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Help line: 1-410-436-4484 or (Edgewood Ops Center - for military use) DSN 584-4484 USAMRIID Emergency Response Line: 1-888-872-7443 CDC'S Bioterrorism Preparedness and Response Center: 1-770-488-7100 John's Hopkins Center for Civilian Biodefense: 1-410-223-1667 (Civilian Biodefense Studies) An Adobe Acrobat Reader (pdf file) version and a Palm OS Electronic version of this Handbook can both be downloaded from the Internet at: http://www.usamriid.army.mil/education/bluebook.html 2 USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Fourth Edition February 2001 Editors: LTC Mark Kortepeter LTC George Christopher COL Ted Cieslak CDR Randall Culpepper CDR Robert Darling MAJ Julie Pavlin LTC John Rowe COL Kelly McKee, Jr. COL Edward Eitzen, Jr. Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 3 PREFACE TO THE FOURTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. Since the first edition in 1993, the awareness of biological weapons in the United States has increased dramatically. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -
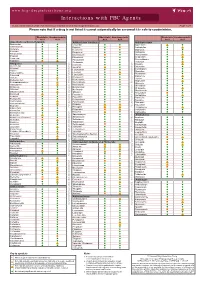
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

Part I Biopharmaceuticals
1 Part I Biopharmaceuticals Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 3 1 Analogs and Antagonists of Male Sex Hormones Robert W. Brueggemeier The Ohio State University, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Columbus, Ohio 43210, USA 1Introduction6 2 Historical 6 3 Endogenous Male Sex Hormones 7 3.1 Occurrence and Physiological Roles 7 3.2 Biosynthesis 8 3.3 Absorption and Distribution 12 3.4 Metabolism 13 3.4.1 Reductive Metabolism 14 3.4.2 Oxidative Metabolism 17 3.5 Mechanism of Action 19 4 Synthetic Androgens 24 4.1 Current Drugs on the Market 24 4.2 Therapeutic Uses and Bioassays 25 4.3 Structure–Activity Relationships for Steroidal Androgens 26 4.3.1 Early Modifications 26 4.3.2 Methylated Derivatives 26 4.3.3 Ester Derivatives 27 4.3.4 Halo Derivatives 27 4.3.5 Other Androgen Derivatives 28 4.3.6 Summary of Structure–Activity Relationships of Steroidal Androgens 28 4.4 Nonsteroidal Androgens, Selective Androgen Receptor Modulators (SARMs) 30 4.5 Absorption, Distribution, and Metabolism 31 4.6 Toxicities 32 Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 4 Analogs and Antagonists of Male Sex Hormones 5 Anabolic Agents 32 5.1 Current Drugs on the Market 32 5.2 Therapeutic Uses and Bioassays -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object.