Medical Management of Biological Casualties Handbook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Microscopic Threat to the United States: Biological Weapons, Biological Terrorism, and Their Multifaceted Implications for U.S
“THE MICROSCOPIC THREAT TO THE UNITED STATES: BIOLOGICAL WEAPONS, BIOLOGICAL TERRORISM, AND THEIR MULTIFACETED IMPLICATIONS FOR U.S. SECURITY” by Angelina Camacho A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts in Global Security Studies Baltimore, Maryland May, 2014 © 2014 Angelina Camacho All Rights Reserved ABSTRACT From 1943 to 1969 the United States had a thriving biological weapons program to develop new ways of targeting its adversaries. With the 1972 creation of the Biological Weapons Convention, the United States relinquished its program and sought to prevent other countries from possessing these lethal weapons. While previously the United States mainly worked with other states and the international community to minimize the threat from biological weapons, the 2001 anthrax attacks changed this landscape by adding a domestic dimension. This thesis explores three major aspects of the biological threat to the United States: domestic lone wolf actors, possible future state threats, and the failing aspects of the Biological Weapons Convention. An analysis of each aspect of the biological threat is performed to identify the role they each may play in future U.S. security decisions. Among the multitude of threats that can arise from biological terrorism and weapons, these particular threats are the most likely to shape future U.S. decision making, both domestically and at the international level. Through an analysis of a specific aspect of the biological threat towards the United States, each chapter illustrates the biological threat to the United States is real, menacing, and must be addressed for the future of U.S. -

Equine Biosecurity and Biocontainment Practices on U.S
Veterinary Services Centers for Epidemiology and Animal Health November 2006 _________________________________________________________________________________________________________________________ Equine Biosecurity and Biocontainment Practices on U.S. Equine Operations With the globalization of animal industries, biosecurity General Practices and biocontainment practices play a vital role in the health of domestic animals. Biosecurity practices include General biosecurity measures taken on equine measures that reduce the risk of disease introduction on operations include isolation protocols, infection-control an operation. Biocontainment practices include precautions for visitors, and use of separate or measures that reduce the spread of disease on an disinfected equipment. Other general management operation and from one operation to another. practices address contact with other animals, potential Vaccination, another important aid in infection control, contamination of feed and water, insect control, and will be addressed in a separate information sheet. manure management. Equine diseases are spread in a variety of ways, including direct contact between equids and contact with Separation for isolation or infection control feces, insect or animal vectors, aerosol particles, and contaminated needles. Often overlooked but equally Overall, 65.1 percent of operations separated important modes of disease transmission include contact animals for isolation or infection control. For this study, with contaminated equipment, tack, transport -

Use of Unapproved Products, Off-Label Use, and Black-Box Warning
Use of Unapproved Products, Off-Label Use, and Black-Box Warning ... A Variation of Newton’s Third Law or the Practical Application of the Rule of Unin- tended Consequences … Considerations in Military Operational Medicine Jerome F. Pierson, RPh, PhD Executive Editors Note: As multiple medical research and development efforts (both in SOF and in conventional research units) offer new drugs and medical equipment to the battlefield, we need to understand the rules of engagement for use, which, are dif- ferent for individual providers versus military heath care systems. ABSTRACT This article discusses the regulatory requirements for the use of unapproved drugs and off-label use of drugs and provides specific examples for military medicine. Additionally, it explains issues associated with standardization by the Service as a designated set, kit, or outfit as opposed to a general guidance. The former situation can be interpreted as a de facto policy whereas the latter is an adaptation of practice of medicine. Recent news stories brought to light the challenges assaulted from many sides to include consumer groups who facing military medicine in an environment where dramatic at times advocate for greater safety and at other times de- life-saving measures have become the expected rather then mand quicker access to therapeutic breakthroughs as well the exception.1,2 However, many of these same life-saving as the pharmaceutical industry which faces demands from measures have the potential to place a practitioner in situa- stockholders for return on investment. Reliance upon re- tions of “damned if you do, damned if you don’t” with re- sults from controlled clinical trials is problematic in that it gard to adherence to various regulatory and legal hurdles. -

112. Na Na Na 112. Only You Remix 112. Come See Me Remix 12
112. Na Na Na 112. Only You Remix 112. Come See Me Remix 12 Gauge Dunkie Butt 12 Gauge Dunkie Butt 2 Live Crew Sports Weekend 2nd II None If You Want It 2nd II None Classic 220 2Pac Changes 2Pac All Eyez On Me 2Pac All Eyez On Me 2Pac I Get Around / Keep Ya Head Up 50 Cent Candy Shop 702. Where My Girls At 7L & Esoteric The Soul Purpose A Taste Of Honey A Taste Of Honey A Tribe Called Quest Unreleased & Unleashed Above The Law Untouchable Abyssinians Best Of The Abyssinians Abyssinians Satta Adele 21. Adele 21. Admiral Bailey Punanny ADOR Let It All Hang Out African Brothers Hold Tight (colorido) Afrika Bambaataa Renegades Of Funk Afrika Bambaataa Planet Rock The Album Afrika Bambaataa Planet Rock The Album Agallah You Already Know Aggrolites Rugged Road (vinil colorido) Aggrolites Rugged Road (vinil colorido) Akon Konvicted Akrobatik The EP Akrobatik Absolute Value Al B. Sure Rescue Me Al Green Greatest Hits Al Johnson Back For More Alexander O´Neal Criticize Alicia Keys Fallin Remix Alicia Keys As I Am (vinil colorido) Alicia Keys A Woman´s Worth Alicia Myers You Get The Best From Me Aloe Blacc Good Things Aloe Blacc I Need A Dollar Alpha Blondy Cocody Rock Althea & Donna Uptown Top Ranking (vinil colorido) Alton Ellis Mad Mad Amy Winehouse Back To Black Amy Winehouse Back To Black Amy Winehouse Lioness : The Hidden Treasures Amy Winehouse Lioness : The Hidden Treasures Anita Baker Rapture Arthur Verocai Arthur Verocai Arthur Verocai Arthur Verocai Augustus Pablo King Tubby Meets Rockers Uptown Augustus Pablo In Fine Style Augustus Pablo This Is Augustus Pablo Augustus Pablo Dubbing With The Don Augustus Pablo Skanking Easy AZ Sugar Hill B.G. -

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

King and Country: Shakespeare’S Great Cycle of Kings Richard II • Henry IV Part I Henry IV Part II • Henry V Royal Shakespeare Company
2016 BAM Winter/Spring #KingandCountry Brooklyn Academy of Music Alan H. Fishman, Chairman of the Board William I. Campbell, Vice Chairman of the Board BAM, the Royal Shakespeare Company, and Adam E. Max, Vice Chairman of the Board The Ohio State University present Katy Clark, President Joseph V. Melillo, Executive Producer King and Country: Shakespeare’s Great Cycle of Kings Richard II • Henry IV Part I Henry IV Part II • Henry V Royal Shakespeare Company BAM Harvey Theater Mar 24—May 1 Season Sponsor: Directed by Gregory Doran Set design by Stephen Brimson Lewis Global Tour Premier Partner Lighting design by Tim Mitchell Music by Paul Englishby Leadership support for King and Country Sound design by Martin Slavin provided by the Jerome L. Greene Foundation. Movement by Michael Ashcroft Fights by Terry King Major support for Henry V provided by Mark Pigott KBE. Major support provided by Alan Jones & Ashley Garrett; Frederick Iseman; Katheryn C. Patterson & Thomas L. Kempner Jr.; and Jewish Communal Fund. Additional support provided by Mercedes T. Bass; and Robert & Teresa Lindsay. #KingandCountry Royal Shakespeare Company King and Country: Shakespeare’s Great Cycle of Kings BAM Harvey Theater RICHARD II—Mar 24, Apr 1, 5, 8, 12, 14, 19, 26 & 29 at 7:30pm; Apr 17 at 3pm HENRY IV PART I—Mar 26, Apr 6, 15 & 20 at 7:30pm; Apr 2, 9, 23, 27 & 30 at 2pm HENRY IV PART II—Mar 28, Apr 2, 7, 9, 21, 23, 27 & 30 at 7:30pm; Apr 16 at 2pm HENRY V—Mar 31, Apr 13, 16, 22 & 28 at 7:30pm; Apr 3, 10, 24 & May 1 at 3pm ADDITIONAL CREATIVE TEAM Company Voice -
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
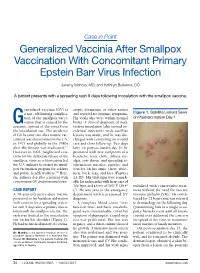
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

Free Executive Summary
Giving Full Measure to Countermeasures: Addressing Problems in the DoD Program to Develop Medical Countermeasures Against Biological Warfare Agents http://www.nap.edu/catalog/10908.html Committee on Accelerating the Research, Development, and Acquisition of Medical Countermeasures Against Biological Warfare Agents Medical Follow-up Agency and Board on Life Sciences Lois M. Joellenbeck, Jane S. Durch, and Leslie Z. Benet, Editors Copyright © National Academy of Sciences. All rights reserved. Giving Full Measure to Countermeasures: Addressing Problems in the DoD Program to Develop Medical Countermeasures Against Biological Warfare Agents http://www.nap.edu/catalog/10908.html THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the Na- tional Academy of Sciences, the National Academy of Engineering, and the Institute of Medi- cine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. Support for this project was provided by Contract No. DAMD17-02-C-0099 between the National Academy of Sciences and the U.S. Army. The views presented in this report are those of the Institute of Medicine and National Research Council Committee on Accelerat- ing the Research, Development, and Acquisition of Medical Countermeasures Against Bio- logical Warfare Agents and are not necessarily those of the funding agencies. Library of Congress Cataloging-in-Publication Data Giving full measure to countermeasures : addressing problems in the DOD program to de- velop medical countermeasures against biological warfare agents / Committee on Acceler- ating the Research, Development, and Acquisition of Medical Countermeasures against Bio- logical Warfare Agents, Medical Follow-up Agency and Board on Life Sciences ; Lois M. -

Medical Management of Biologic Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Fourth Edition February 2001 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES ¨ FORT DETRICK FREDERICK, MARYLAND 1 Sources of information: National Response Center 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Help line: 1-410-436-4484 or (Edgewood Ops Center - for military use) DSN 584-4484 USAMRIID Emergency Response Line: 1-888-872-7443 CDC'S Bioterrorism Preparedness and Response Center: 1-770-488-7100 John's Hopkins Center for Civilian Biodefense: 1-410-223-1667 (Civilian Biodefense Studies) An Adobe Acrobat Reader (pdf file) version and a Palm OS Electronic version of this Handbook can both be downloaded from the Internet at: http://www.usamriid.army.mil/education/bluebook.html 2 USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Fourth Edition February 2001 Editors: LTC Mark Kortepeter LTC George Christopher COL Ted Cieslak CDR Randall Culpepper CDR Robert Darling MAJ Julie Pavlin LTC John Rowe COL Kelly McKee, Jr. COL Edward Eitzen, Jr. Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 3 PREFACE TO THE FOURTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. Since the first edition in 1993, the awareness of biological weapons in the United States has increased dramatically. -

Communicable Disease Toolkit Liberia
WHO/CDS/2004.24 COMMUNICABLE DISEASE TOOLKIT LIBERIA 1. COMMUNICABLE DISEASE PROFILE World Health Organization 2004 Communicable Disease Working Group on Emergencies, WHO/HQ The WHO Regional Office for Africa (AFRO) WHO Office, Liberia © World Health Organization 2004 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. Further information is available at: CDS Information Resource Centre, World Health Organization, 1211 Geneva 27, Switzerland; fax: (+41) 22 791 4285, e-mail: [email protected] Communicable Disease Toolkit for LIBERIA 2004: Communicable Disease Profile. ACKNOWLEDGMENTS Edited by Dr Michelle Gayer, Dr Katja Schemionek, Dr Monica Guardo, and Dr Máire Connolly of the Programme on Communicable Diseases in Complex Emergencies, WHO/CDS. This Profile is a collaboration between the Communicable Disease Working Group on Emergencies (CD-WGE) at WHO/HQ, the Division of Communicable Disease Prevention and Control (DCD) at WHO/AFRO and the Office of the WHO Representative for Liberia. -

Concepts of Biocontainment & Biosafety Training
Concepts of Biocontainment & Biosafety Training UNIVERSITY OF NORTH CAROLINA - CHARLOTTE/ APRIL 30, 2014 CLIENT NAME / DATE Regulatory Guidance . OSHA Bloodborne Pathogen Standard . NIH Recombinant Guidelines – Apply to all institutions receiving NIH funding – Covers rDNA work, but also includes risk group listing . Biosafety in Microbiological and Biomedical Laboratories (BMBL) . Select Agent and Toxin regulations . Dangerous goods shipping regulations . North Carolina Dept. of Environment and Natural Resources CLIENT NAME / DATE US Guidance for Laboratories . BMBL Outlines: – Standard Microbiological Practices – Special Microbiological Practices – Safety Equipment – Facilities . Includes – Agent summary statements – BSC design and use – Toxin handling http://www.cdc.gov/biosafety/publications/bmbl5/index.htm CLIENT NAME / DATE Layers of Protection CLIENT NAME / DATE Definitions . Biohazard – A biological agent capable of self- replication that can cause disease in humans, animals, or plants – Generally, a microorganism, or toxins and allergens derived from those organisms . Biocontainment Class III cabinets at the U.S. Biological Warfare Laboratories, Camp Detrick, Maryland (Photo, 1940s) – the physical containment of pathogenic organisms or agents to prevent accidental infection of workers or release into the surrounding community . CLIENT NAME / DATE Biosafety . Fundamental objective: – Containment of potentially harmful biological agents . Risk assessment: – Helps to assign the biosafety level that reduces to an absolute minimum the worker’s exposure to agents, their risk of an LAI (lab associated infection), and potential impact on the community and the environment CLIENT NAME / DATE Biosafety Levels (BSLs) . Combinations of lab practices and techniques, safety equipment, and laboratory facilities . Appropriate level determined by risk assessment . Specifically appropriate for: – Organism in use – Operations performed in the laboratory – Known or suspected routes of infection CLIENT NAME / DATE Determining BSLs . -

Dengue and Yellow Fever
GBL42 11/27/03 4:02 PM Page 262 CHAPTER 42 Dengue and Yellow Fever Dengue, 262 Yellow fever, 265 Further reading, 266 While the most important viral haemorrhagic tor (Aedes aegypti) as well as reinfestation of this fevers numerically (dengue and yellow fever) are insect into Central and South America (it was transmitted exclusively by arthropods, other largely eradicated in the 1960s). Other factors arboviral haemorrhagic fevers (Crimean– include intercontinental transport of car tyres Congo and Rift Valley fevers) can also be trans- containing Aedes albopictus eggs, overcrowding mitted directly by body fluids. A third group of of refugee and urban populations and increasing haemorrhagic fever viruses (Lassa, Ebola, Mar- human travel. In hyperendemic areas of Asia, burg) are only transmitted directly, and are not disease is seen mainly in children. transmitted by arthropods at all. The directly Aedes mosquitoes are ‘peri-domestic’: they transmissible viral haemorrhagic fevers are dis- breed in collections of fresh water around the cussed in Chapter 41. house (e.g. water storage jars).They feed on hu- mans (anthrophilic), mainly by day, and feed re- peatedly on different hosts (enhancing their role Dengue as vectors). Dengue virus is numerically the most important Clinical features arbovirus infecting humans, with an estimated Dengue virus may cause a non-specific febrile 100 million cases per year and 2.5 billion people illness or asymptomatic infection, especially in at risk.There are four serotypes of dengue virus, young children. However, there are two main transmitted by Aedes mosquitoes, and it is un- clinical dengue syndromes: dengue fever (DF) usual among arboviruses in that humans are the and dengue haemorrhagic fever (DHF).