Vinorelbine Inj. USP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
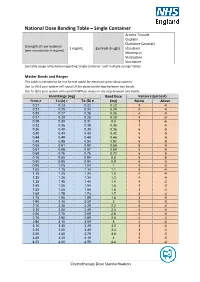
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

Vincristine (Conventional): Drug Information
Official reprint from UpToDate® www.uptodate.com ©2017 UpToDate® Vincristine (conventional): Drug information Copyright 1978-2017 Lexicomp, Inc. All rights reserved. (For additional information see "Vincristine (conventional): Patient drug information" and see "Vincristine (conventional): Pediatric drug information") For abbreviations and symbols that may be used in Lexicomp (show table) Special Alerts Vincristine Sulfate Safety Alert October 2015 Health Canada is notifying health care providers that certain lots of Hospira’s vincristine sulfate 1 mg/mL injection (DIN 02183013: 2 mL vial, list #7077A001; 5 mL vial, list #7082A001) have incorrect or outdated safety information on the inner/outer labels and package insert, which may increase the risk to patients and may result in significant patient harm requiring medical intervention. These warnings include: - Vincristine should only be administered by the intravenous (IV) route. Administration of vincristine by any other route can be fatal. - Syringes containing this product should be labeled “Warning - for IV use only.” - Extemporaneously prepared syringes containing this product must be packaged in an overwrap which is labeled “Do not remove covering until moment of injection. For IV use only - fatal if given by other routes.” - Contraindication of vincristine in patients with demyelinating Charcot-Marie-Tooth syndrome. - Potential risk of acute shortness of breath when vincristine is coadministered with mitomycin-C and GI toxicities including necrosis with administration of vincristine. Health care providers are requested to consult with the approved Canadian product monograph for vincristine sulfate 1 mg/mL for the most updated information. Consumers with questions should contact their health care provider for more information. ALERT: US Boxed Warning Experienced physician: Vincristine should be administered by individuals experienced in the administration of the drug. -

Effect of Human Fibroblast Interferon on the Antiviral Activity of Mammalian Cells Treated with Bleomycin, Vincristine, Or Mitomycin C1
[CANCER RESEARCH 43, 5462-5466. November 1983] Effect of Human Fibroblast Interferon on the Antiviral Activity of Mammalian Cells Treated with Bleomycin, Vincristine, or Mitomycin C1 Robert J. Suhadolnik,2 Yosuke Sawada,3 Maryann B. Flick, Nancy L. Reichenbach, and Joseph D. Mosca3 Department of Biochemistry, Temple University School of Medicine, Philadelphia, Pennsylvania 19140 ABSTRACT protein kinase (2,9,28,34,36). In addition to the use of interferon in the treatment of cancer (1, 11, 12, 29, 34), combination Bleomycin, vincristine, or mitomycin C, when added to HeLa chemotherapy of interferon and methotrexate, c/s-platinum diam- cells simultaneously with human fibroblast interferon (IFN-0), minedichloride, cyclophosphamide, or 1,3-bis(/i-chloroethyl)-1- caused a decrease in cell density and inhibited DMA synthesis nitrosourea on either tumor cells in culture or in leukemic mice compared with HeLa cells treated with IFN-/3 alone. However, has been reported (5, 7, 10, 25). Furthermore, Stolfi ef al. (37) the IFN-0-induced antiviral processes were unaffected by the reported recently that the administration of mouse interferon to presence of these drugs as determined by in vitro enzyme assays mice following the administration of 5-fluorouracil protected the and the development of the antiviral state in the intact HeLa cell. mice from mortality. Because it is possible to selectively inhibit HeLa cells treated with IFN-/Õalone or with IFN-/3 in combination proliferation of tumor cells with chemotherapeutic drugs, we with bleomycin, vincristine, or mitomycin C were able to induce reasoned that the ability of the normal cell to maintain the antiviral the double-stranded RNA-dependent adenosine triphos- phate:2',5'-oligoadenylic acid adenyltransferase (EC 2.2.2.-) and state might be adversely affected by such drugs and that the antiproliferative action of interferons might affect the activity of the double-stranded RNA-dependent protein kinase. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Metals and Metal Compounds in Cancer Treatment
ANTICANCER RESEARCH 24: 1529-1544 (2004) Review Metals and Metal Compounds in Cancer Treatment BERNARD DESOIZE Laboratoire de Biochimie et de Biologie Moléculaire, EA 3306, IFR 53, Faculté de Pharmacie, 51 rue Cognacq-Jay, 51096 Reims cedex, France Abstract. Metals and metal compounds have been used in The importance of metal compounds in medicine is medicine for several thousands of years. In this review we undisputed, as can be judged by the use of compounds of summarized the anti-cancer activities of the ten most active antimony (anti-protozoal), bismuth (anti-ulcer), gold (anti- metals: arsenic, antimony, bismuth, gold, vanadium, iron, arthritic), iron (anti-malarial), silver (anti-microbial) and rhodium, titanium, gallium and platinum. The first reviewed platinum (anti-cancer) in the treatment of various diseases. metal, arsenic, presents the anomaly of displaying anti-cancer In terms of anti-tumour activity, a wide range of compounds and oncogenic properties simultaneously. Some antimony of both transition metals and main group elements have derivatives, such as Sb2O3, salt (tartrate) and organic been investigated for efficacy (1). The earliest reports on the compounds, show interesting results. Bismuth directly affects therapeutic use of metals or metal-containing compounds in Helicobacter pylori and gastric lymphoma; the effects of cancer and leukemia date from the sixteenth century. bismuth complexes of 6-mercaptopurine are promising. Superoxide dismutase inhibition leads to selective killing Gold(I) and (III) compounds show anti-tumour activities, of cancer cells in vitro and in vivo (2,3). As a consequence, although toxicity remains high. Research into the potential use reactive oxygen species (ROS) may not only be involved in of gold derivatives is still ongoing. -

Eliminating Vincristine Administration Events
Issue 37 October 2017 Eliminating vincristine administration events Issue: Despite the usually deadly consequences of accidentally administering the chemotherapy drug vincristine intrathecally, adverse events still occur, typically because some organizations still administer vincristine via syringe. The good news is that these events are happening less in the United States, mostly because of the efforts of leading national organizations to promote an effective prevention strategy that assures a mechanical barrier to intrathecal administration (into the subarachnoid space). The strategy involves diluting intravenous vincristine or other vinca alkaloids in a minibag that contains a volume that is too large for intrathecal administration (e.g., 25 mL for pediatric patients and 50 mL for adults), making it mechanically difficult to accidentally administer intrathecally.1 Vinca alkaloids (vinblastine, vinorelbine, vincristine, and vincristine liposomal) are chemotherapy drugs that are intended to be administered intravenously. If given intrathecally, vincristine is nearly always fatal and associated with an irreversible, painful ascending paralysis.2 When vinca alkaloids are injected intrathecally, destruction of the central nervous system occurs, radiating out from the injection site. The few survivors of this adverse event experienced devastating neurological damage.1 Part of the problem stems from ordering intravenous vincristine in conjunction with medications that are administered intrathecally via a syringe, such as methotrexate, cytarabine and hydrocortisone. In some adverse events, vincristine was mistakenly injected into the cerebrospinal fluid (CSF) of patients when the intent was to inject another intrathecal chemotherapy agent, such as methotrexate or cytarabine.3 Intravenous vincristine and other vinca alkaloids are dispensed from the pharmacy with explicit warning labels about their lethality if given intrathecally. -

Vincristine Archive File
Vincristine archive file Vincristine is a vinakaloid, which was first discovered 1958 in the tropical plant Catharanthus roseus, native in Madagascar. Its ability to inhibit the metaphase of the mitosis by suppressing the polymerization of the microtubule makes it very important as a chemotherapeutic agent, especially against non-Hodgkin lymphomas and Wilms' tumor. History: Vinblastine and Vincristine are bisindole alkaloids and both widely known for their use as antitumor drugs. In former times they were isolated in trace quantities from the leaves of Catharanthus roseus. Because of their importance in the medical field numerous researches were examining the structure, use and synthesis of Vinblastine, Vincristine and their derivates. Their biological effect to inhibit the microtubule formation and mitosis was and still is a very important part of medical cancer therapy. They were both among the first natural products whose structures were identified by X-ray crystallography and among the first for which X-ray analysis of a heavy atom derivative was used to establish their absolute configuration. ( 2) Timeline: 1958 Vinblastine was first discovered as an unexpected myelosuppressive agent by Noble, R. L., C. T. Beer, and J. H. Cutts during the search for an antidiabetic agent in Catharanthus roseus (myelosupression decreased activity of the bone marrow) 1959 independendently researchers from Eli Lilly (Johnson, I. S., J. G. Armstrong, M. Gorman, and J. P. Burnett, Jr.)discovered that the extracts of C. roseus Possesses activity against -

Collected Mass Spectrometry Data on Monoterpene Indole Alkaloids from Natural Product Chemistry Research
www.nature.com/scientificdata OPEN Collected mass spectrometry data DATA DescrIPTOR on monoterpene indole alkaloids from natural product chemistry Received: 20 September 2018 Accepted: 25 February 2019 research Published: xx xx xxxx Alexander E. Fox Ramos 1, Pierre Le Pogam1, Charlotte Fox Alcover 1, Elvis Otogo N’Nang 1, Gaëla Cauchie 1, Hazrina Hazni1,2, Khalijah Awang2, Dimitri Bréard3, Antonio M. Echavarren4,5, Michel Frédérich6, Thomas Gaslonde7, Marion Girardot8, Raphaël Grougnet7, Mariia S. Kirillova4, Marina Kritsanida 7, Christelle Lémus7, Anne-Marie Le Ray3, Guy Lewin1, Marc Litaudon9, Lengo Mambu 10, Sylvie Michel7, Fedor M. Miloserdov4, Michael E. Muratore4, Pascal Richomme-Peniguel3, Fanny Roussi9, Laurent Evanno1, Erwan Poupon1, Pierre Champy1 & Mehdi A. Beniddir 1 This Data Descriptor announces the submission to public repositories of the monoterpene indole alkaloid database (MIADB), a cumulative collection of 172 tandem mass spectrometry (MS/MS) spectra from multiple research projects conducted in eight natural product chemistry laboratories since the 1960s. All data have been annotated and organized to promote reuse by the community. Being a unique collection of these complex natural products, these data can be used to guide the dereplication and targeting of new related monoterpene indole alkaloids within complex mixtures when applying computer-based approaches, such as molecular networking. Each spectrum has its own accession number from CCMSLIB00004679916 to CCMSLIB00004680087 on the GNPS. The MIADB is available for download from MetaboLights under the identifer: MTBLS142 (https://www.ebi.ac.uk/metabolights/ MTBLS142). Background & Summary Monoterpene indole alkaloids (MIAs) constitute a broad class of nitrogen-containing plant-derived natural products composed of more than 3000 members1. -

Paclitaxel, Vinorelbine and 5-Fluorouracil in Breast Cancer Patients Pretreated with Adjuvant Anthracyclines
British Journal of Cancer (2005) 92, 634 – 638 & 2005 Cancer Research UK All rights reserved 0007 – 0920/05 $30.00 www.bjcancer.com Paclitaxel, vinorelbine and 5-fluorouracil in breast cancer patients pretreated with adjuvant anthracyclines Clinical Studies 1 1 1 2 2 2 3 3 A Berruti , R Bitossi , G Gorzegno , A Bottini , D Generali , M Milani , D Katsaros , IA Rigault de la Longrais , 3 4 4 4 5 6 6 7 R Bellino , M Donadio , M Ardine , O Bertetto , S Danese , MG Sarobba , A Farris , V Lorusso and ,1 L Dogliotti* 1Oncologia Medica, Azienda Ospedaliera San Luigi, Regione Gonzole 10, 10043 Orbassano (TO), Italy; 2Breast Unit, Azienda Ospedaliera Istituti Ospitalieri, largo Priori, 26100 Cremona, Italy; 3Ginecologia Oncologica, Azienda Ospedaliera OIRM Sant’Anna, via Ventimiglia 3, 10126 Torino, Italy; 4 Oncologia Medica, Centro Oncologico Ematologico Subalpino, Azienda Ospedaliera San Giovanni Battista Molinette, corso Bramante 88, 10126 Torino, 5 6 Italy; Ginecologia Divisione A, Azienda Ospedaliera OIRM Sant’Anna, corso Spezia 60, 10126 Torino, Italy; Oncologia Medica, Istituto Clinica Medica 7 Universitaria, via San Pietro 8, 07100 Sassari, Italy; Oncologia Medica, Istituto Oncologico, via Amendola 209, 70126 Bari, Italy We investigated the activity and toxicity of a combination of vinorelbine (VNB), paclitaxel (PTX) and 5-fluorouracil (5-FU) continuous infusion administered as first-line chemotherapy in metastatic breast cancer patients pretreated with adjuvant À2 À2 anthracyclines. A total of 61 patients received a regimen consisting of VNB 25 mg m on days 1 and 15, PTX 60 mg m on days 1, 8 À2 and 15 and continuous infusion of 5-FU at 200 mg m every day.