Antimicrotubule Effects of Estramustine, an Antiprostatic Tumor Drug
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
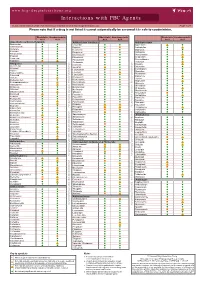
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Order in Council 1243/1995
PROVINCE OF BRITISH COLUMBIA ORDER OF THE LIEUTENANT GOVERNOR IN COUNCIL Order in Council No. 12 4 3 , Approved and Ordered OCT 121995 Lieutenant Governor Executive Council Chambers, Victoria On the recommendation of the undersigned, the Lieutenant Governor, by and with the advice and consent of the Executive Council, orders that Order in Council 1039 made August 17, 1995, is rescinded. 2. The Drug Schedules made by regulation of the Council of the College of Pharmacists of British Columbia, as set out in the attached resolution dated September 6, 1995, are hereby approved. (----, c" g/J1"----c- 4- Minister of Heal fandand Minister Responsible for Seniors Presidin Member of the Executive Council (This pan is for adnwustratlye purposes only and is not part of the Order) Authority under which Order Is made: Act and section:- Pharmacists, Pharmacy Operations and Drug Scheduling Act, section 59(2)(1), 62 Other (specify): - Uppodukoic1enact N6145; Resolution of the Council of the College of Pharmacists of British Columbia ("the Council"), made by teleconference at Vancouver, British Columbia, the 6th day of September 1995. RESOLVED THAT: In accordance with the authority established in Section 62 of the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia, S.B.C. Chapter 62, the Council makes the Drug Schedules by regulation as set out in the attached schedule, subject to the approval of the Lieutenant Governor in Council. Certified a true copy Linda J. Lytle, Phr.) Registrar DRUG SCHEDULES to the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia The Drug Schedules have been printed in an alphabetical format to simplify the process of locating each individual drug entry and determining its status in British Columbia. -

Emcyt® Estramustine Phosphate Sodium Capsules DESCRIPTION
Emcyt® estramustine phosphate sodium capsules DESCRIPTION Estramustine phosphate sodium, an antineoplastic agent, is an off-white powder readily soluble in water. EMCYT Capsules are white and opaque, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate, for oral administration. Each capsule also contains magnesium stearate, silicon dioxide, sodium lauryl sulfate, and talc. Gelatin capsule shells contain the following pigment: titanium dioxide. Chemically, estramustine phosphate sodium is estra-1,3,5(10)-triene-3,17-diol(17ß)-,3 [bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. It is also referred to as estradiol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. Estramustine phosphate sodium has an empiric formula of C23H30Cl2NNa2O6P•H2O, a calculated molecular weight of 582.4, and the following structural formula: CLINICAL PHARMACOLOGY Estramustine phosphate (Figure 1) is a molecule combining estradiol and nornitrogen mustard by a carbamate link. The molecule is phosphorylated to make it water soluble. 1 Estramustine phosphate taken orally is readily dephosphorylated during absorption, and the major metabolites in plasma are estramustine (Figure 2), the estrone analog (Figure 3), estradiol, and estrone. Prolonged treatment with estramustine phosphate produces elevated total plasma concentrations of estradiol that fall within ranges similar to the elevated estradiol levels found in prostatic cancer patients given conventional estradiol therapy. Estrogenic effects, as demonstrated by changes in circulating levels of steroids and pituitary hormones, are similar in patients treated with either estramustine phosphate or conventional estradiol. 2 The metabolic urinary patterns of the estradiol moiety of estramustine phosphate and estradiol itself are very similar, although the metabolites derived from estramustine phosphate are excreted at a slower rate. -

2021 Formulary List of Covered Prescription Drugs
2021 Formulary List of covered prescription drugs This drug list applies to all Individual HMO products and the following Small Group HMO products: Sharp Platinum 90 Performance HMO, Sharp Platinum 90 Performance HMO AI-AN, Sharp Platinum 90 Premier HMO, Sharp Platinum 90 Premier HMO AI-AN, Sharp Gold 80 Performance HMO, Sharp Gold 80 Performance HMO AI-AN, Sharp Gold 80 Premier HMO, Sharp Gold 80 Premier HMO AI-AN, Sharp Silver 70 Performance HMO, Sharp Silver 70 Performance HMO AI-AN, Sharp Silver 70 Premier HMO, Sharp Silver 70 Premier HMO AI-AN, Sharp Silver 73 Performance HMO, Sharp Silver 73 Premier HMO, Sharp Silver 87 Performance HMO, Sharp Silver 87 Premier HMO, Sharp Silver 94 Performance HMO, Sharp Silver 94 Premier HMO, Sharp Bronze 60 Performance HMO, Sharp Bronze 60 Performance HMO AI-AN, Sharp Bronze 60 Premier HDHP HMO, Sharp Bronze 60 Premier HDHP HMO AI-AN, Sharp Minimum Coverage Performance HMO, Sharp $0 Cost Share Performance HMO AI-AN, Sharp $0 Cost Share Premier HMO AI-AN, Sharp Silver 70 Off Exchange Performance HMO, Sharp Silver 70 Off Exchange Premier HMO, Sharp Performance Platinum 90 HMO 0/15 + Child Dental, Sharp Premier Platinum 90 HMO 0/20 + Child Dental, Sharp Performance Gold 80 HMO 350 /25 + Child Dental, Sharp Premier Gold 80 HMO 250/35 + Child Dental, Sharp Performance Silver 70 HMO 2250/50 + Child Dental, Sharp Premier Silver 70 HMO 2250/55 + Child Dental, Sharp Premier Silver 70 HDHP HMO 2500/20% + Child Dental, Sharp Performance Bronze 60 HMO 6300/65 + Child Dental, Sharp Premier Bronze 60 HDHP HMO -

Binding Characteristics of a Major Protein in Rat Ventral Prostate Cytosol That Interacts with Estramustine, a Nitrogen Mustard Derivative of 17ß-Estradiol
[CANCER RESEARCH 39, 5155-5164, December 1979] 0008-5472/79/0039-OOOOS02.00 Binding Characteristics of a Major Protein in Rat Ventral Prostate Cytosol That Interacts with Estramustine, a Nitrogen Mustard Derivative of 17ß-Estradiol BjörnForsgren, Jan-Àke Gustafsson, Ake Pousette, and Bertil Hogberg AB Leo Research Laboratories. Pack. S-251 00 Helsingborg, Sweden [B.F.. B.H.], and Departments of Chemistry and Medical Nutrition. ¡J-ÄG.. A P.¡and Pharmacology [BH.], Karolinska Institute!. S-104 01 Stockholm 60. Sweden ABSTRACT causes atrophy of the testes and accessory sex organs; re duces the uptake of zinc by the prostate; depresses the 5a- The tissue distribution of [3H]estramustine, the dephospho- reductase, arginase, and acid phosphatase activities in the rylated metabolite of estramustine phosphate (Estracyt), in the male rat was compared to that of [3H]estradiol 30 min and 2 hr prostate; and affects lipid and carbohydrate metabolism (10, 11, 20, 29, 31 -33, 42). Although the observed effects in many following i.p. administration. In contrast to estradiol, estramus respects are similar to estrogenic effects, several experimental tine was found to be efficiently concentrated in the ventral and clinical results indicate that estramustine phosphate affects prostate gland by a soluble protein. The binding characteristics normal and neoplastic prostate tissue in a way that cannot be of this protein were studied in vitro using cytosol preparations attributed solely to its antigonadotropic or weak estrogenic of the gland. With a dextran-coated charcoal technique, the properties (15, 16, 30, 38). protein was found to bind estramustine with a broad pH opti Plym Forshell and Nilsson (35), using labeled compounds (1 mum between pH 7 and pH 8.5, with an apparent Kd of 10 to to 10 mg/kg body weight), found considerably higher levels of 30 nw, and with a binding capacity of about 5 nmol/mg cytosol radioactivity in the rat ventral prostate following i.v. -

OUH Formulary Approved for Use in Breast Surgery
Oxford University Hospitals NHS Foundation Trust Formulary FORMULARY (Y): the medicine can be used as per its licence. RESTRICTED FORMULARY (R): the medicine can be used as per the agreed restriction. NON-FORMULARY (NF): the medicine is not on the formulary and should not be used unless exceptional approval has been obtained from MMTC. UNLICENSED MEDICINE – RESTRICTED FORMULARY (UNR): the medicine is unlicensed and can be used as per the agreed restriction. SPECIAL MEDICINE – RESTRICTED FORMULARY (SR): the medicine is a “special” (unlicensed) and can be used as per the agreed restriction. EXTEMPORANEOUS PREPARATION – RESTRICTED FORMULARY (EXTR): the extemporaneous preparation (unlicensed) can be prepared and used as per the agreed restriction. UNLICENSED MEDICINE – NON-FORMULARY (UNNF): the medicine is unlicensed and is not on the formulary. It should not be used unless exceptional approval has been obtained from MMTC. SPECIAL MEDICINE – NON-FORMULARY (SNF): the medicine is a “special” (unlicensed) and is not on the formulary. It should not be used unless exceptional approval has been obtained from MMTC. EXTEMPORANEOUS PREPARATION – NON-FORMULARY (EXTNF): the extemporaneous preparation (unlicensed) cannot be prepared and used unless exceptional approval has been obtained from MMTC. CLINICAL TRIALS (C): the medicine is clinical trial material and is not for clinical use. NICE TECHNOLOGY APPRAISAL (NICETA): the medicine has received a positive appraisal from NICE. It will be available on the formulary from the day the Technology Appraisal is published. Prescribers who wish to treat patients who meet NICE criteria, will have access to these medicines from this date. However, these medicines will not be part of routine practice until a NICE TA Implementation Plan has been presented and approved by MMTC (when the drug will be given a Restricted formulary status). -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Pharmaceutical Composition and Dosage Forms for Administration of Hydrophobic Drugs
(19) & (11) EP 2 246 049 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 03.11.2010 Bulletin 2010/44 A61K 31/355 (2006.01) A61K 31/56 (2006.01) C07J 53/00 (2006.01) (21) Application number: 10173114.9 (22) Date of filing: 24.05.2004 (84) Designated Contracting States: • Fikstad, David T. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Salt Lake City, UT 84102 (US) HU IE IT LI LU MC NL PL PT RO SE SI SK TR • Zhang, Huiping Salt Lake City, UT 844121 (US) (30) Priority: 22.05.2003 US 444935 • Giliyar, Chandrashekar Salt Lake City, UT 84102 (US) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (74) Representative: Walker, Ross Thomson 04753162.9 / 1 624 855 Potts, Kerr & Co. 15 Hamilton Square (71) Applicant: Lipocine, Inc. Birkenhead Salt Lake City, UT 84103 (US) Merseyside CH41 6BR (GB) (72) Inventors: Remarks: • Chen, Feng-Jing This application was filed on 17-08-2010 as a Salt Lake City, UT 84111 (US) divisional application to the application mentioned • Patel, Mahesh V. under INID code 62. Salt Lake City, UT 84124 (US) (54) Pharmaceutical composition and dosage forms for administration of hydrophobic drugs (57) Pharmaceutical compositions and dosage tion with improved dispersion of both the active agent forms for administration of hydrophobic drugs, particu- and the solubilizer. As a result of the improved dispersion, larly steroids, are provided. The pharmaceutical compo- the pharmaceutical composition has improved bioavail- sitions include a therapeutically effective amount of a hy- ability upon administration. -

Anatomical Classification Guidelines V2021 EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021 Anatomical Classification Guidelines V2021 "The Anatomical Classification of Pharmaceutical Products has been developed and maintained by the European Pharmaceutical Marketing Research Association (EphMRA) and is therefore the intellectual property of this Association. EphMRA's Classification Committee prepares the guidelines for this classification system and takes care for new entries, changes and improvements in consultation with the product's manufacturer. The contents of the Anatomical Classification of Pharmaceutical Products remain the copyright to EphMRA. Permission for use need not be sought and no fee is required. We would appreciate, however, the acknowledgement of EphMRA Copyright in publications etc. Users of this classification system should keep in mind that Pharmaceutical markets can be segmented according to numerous criteria." © EphMRA 2021 Anatomical Classification Guidelines V2021 CONTENTS PAGE INTRODUCTION A ALIMENTARY TRACT AND METABOLISM 1 B BLOOD AND BLOOD FORMING ORGANS 28 C CARDIOVASCULAR SYSTEM 36 D DERMATOLOGICALS 51 G GENITO-URINARY SYSTEM AND SEX HORMONES 58 H SYSTEMIC HORMONAL PREPARATIONS (EXCLUDING SEX HORMONES) 68 J GENERAL ANTI-INFECTIVES SYSTEMIC 72 K HOSPITAL SOLUTIONS 88 L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS 96 M MUSCULO-SKELETAL SYSTEM 106 N NERVOUS SYSTEM 111 P PARASITOLOGY 122 R RESPIRATORY SYSTEM 124 S SENSORY ORGANS 136 T DIAGNOSTIC AGENTS 143 V VARIOUS 145 Anatomical Classification Guidelines V2021 INTRODUCTION The Anatomical Classification was initiated in 1971 by EphMRA. It has been developed jointly by Intellus/PBIRG and EphMRA. It is a subjective method of grouping certain pharmaceutical products and does not represent any particular market, as would be the case with any other classification system. -

PHARMACEUTICAL APPENDIX to the HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev
Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM GADOCOLETICUM 280776-87-6 ABAFUNGIN 129639-79-8 ACIDUM LIDADRONICUM 63132-38-7 ABAMECTIN 65195-55-3 ACIDUM SALCAPROZICUM 183990-46-7 ABANOQUIL 90402-40-7 ACIDUM SALCLOBUZICUM 387825-03-8 ABAPERIDONUM 183849-43-6 ACIFRAN 72420-38-3 ABARELIX 183552-38-7 ACIPIMOX 51037-30-0 ABATACEPTUM 332348-12-6 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABETIMUSUM 167362-48-3 ACIVICIN 42228-92-2 ABIRATERONE 154229-19-3 ACLANTATE 39633-62-0 ABITESARTAN 137882-98-5 ACLARUBICIN 57576-44-0 ABLUKAST 96566-25-5 ACLATONIUM NAPADISILATE 55077-30-0 ABRINEURINUM 178535-93-8 ACODAZOLE 79152-85-5 ABUNIDAZOLE 91017-58-2 ACOLBIFENUM 182167-02-8 ACADESINE 2627-69-2 ACONIAZIDE 13410-86-1 ACAMPROSATE