Environmentally Classified Pharmaceuticals 2014-2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeting Fibrosis in the Duchenne Muscular Dystrophy Mice Model: an Uphill Battle
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Targeting fibrosis in the Duchenne Muscular Dystrophy mice model: an uphill battle 2 Marine Theret1#, Marcela Low1#, Lucas Rempel1, Fang Fang Li1, Lin Wei Tung1, Osvaldo 3 Contreras3,4, Chih-Kai Chang1, Andrew Wu1, Hesham Soliman1,2, Fabio M.V. Rossi1 4 1School of Biomedical Engineering and the Biomedical Research Centre, Department of Medical 5 Genetics, 2222 Health Sciences Mall, Vancouver, BC, V6T 1Z3, Canada 6 2Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Minia 7 University, Minia, Egypt 8 3Developmental and Stem Cell Biology Division, Victor Chang Cardiac Research Institute, 9 Darlinghurst, NSW, 2010, Australia 10 4Departamento de Biología Celular y Molecular and Center for Aging and Regeneration (CARE- 11 ChileUC), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, 8331150 12 Santiago, Chile 13 # Denotes Co-first authorship 14 15 Keywords: drug screening, fibro/adipogenic progenitors, fibrosis, repair, skeletal muscle. 16 Correspondence to: 17 Marine Theret 18 School of Biomedical Engineering and the Biomedical Research Centre 19 University of British Columbia 20 2222 Health Sciences Mall, Vancouver, British Columbia 21 Tel: +1(604) 822 0441 fax: +1(604) 822 7815 22 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Country Reports Japan International Corporation of Welfare Services (JICWELS) Contents 1. Cambodia 1 2. Indonesia 70 3. Malaysia 91 4. Philippines 116 5. Sri Lanka 141 6. Thailand 161 The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Cambodia -1- KINGDOM OF CAMBODIA Nation Religion King Ministry of Health Department of Drugs and Food Country Report The Study Program on Quality Management of Essential Medicines Good Manufacturing Practice (GMP) and Inspection November 4, 2012 – November 30, 2012 Sponsored by : The Government of Japan Japan International Cooperation Agency (JICA) Department of Drugs and Food Ministry of Health, Cambodia. -2- I- COUNTRY PROFILE -3- A-Geography Cambodia is an agricultural country located in South East Asia which bordering the Gulf of Thailand, between Thailand, Vietnam, and Laos. Its approximate geographical coordinates are 13°N 105°E. Its 2,572 km border is split among Vietnam (1,228 km), Thailand (803 km) and Laos (541 km), as well as 443 km of coastline. Cambodia covers 181,035 square kilometers in the southwestern part of the Indochina, Cambodia lies completely within the tropics; its southernmost points are only slightly more than 10° above the equator. The country is bounded on the north by Thailand and by Laos, on the east and southeast by Vietnam, and on the west by the Gulf of Thailand and by Thailand. It consists of the Tonle Sap Basin and the Mekong Lowlands. To the southeast of this great basin is the Mekong Delta, which extends through Vietnam to the South China Sea. -

Novartis R&D and Investor Update
Novartis AG Investor Relations Novartis R&D and investor update November 5, 2018 Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” “agreement to acquire,” or similar expressions, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this presentation, or regarding potential future revenues from such products, or regarding the proposed acquisition of Endocyte, Inc. (Endocyte) by Novartis including the potential outcome and expected timing for completion of the proposed acquisition, and the potential impact on Novartis of the proposed acquisition, including express or implied discussions regarding potential future sales or earnings of Novartis, and any potential strategic benefits, synergies or opportunities expected as a result of the proposed acquisition. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this presentation will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. -

Endocrine Drugs
PharmacologyPharmacologyPharmacology DrugsDrugs thatthat AffectAffect thethe EndocrineEndocrine SystemSystem TopicsTopicsTopics •• Pituitary Pituitary DrugsDrugs •• Parathyroid/Thyroid Parathyroid/Thyroid DrugsDrugs •• Adrenal Adrenal DrugsDrugs •• Pancreatic Pancreatic DrugsDrugs •• Reproductive Reproductive DrugsDrugs •• Sexual Sexual BehaviorBehavior DrugsDrugs FunctionsFunctionsFunctions •• Regulation Regulation •• Control Control GlandsGlandsGlands ExocrineExocrine EndocrineEndocrine •• Secrete Secrete enzymesenzymes •• Secrete Secrete hormoneshormones •• Close Close toto organsorgans •• Transport Transport viavia bloodstreambloodstream •• Require Require receptorsreceptors NervousNervous EndocrineEndocrine WiredWired WirelessWireless NeurotransmittersNeurotransmitters HormonesHormones ShortShort DistanceDistance LongLong Distance Distance ClosenessCloseness ReceptorReceptor Specificity Specificity RapidRapid OnsetOnset DelayedDelayed Onset Onset ShortShort DurationDuration ProlongedProlonged Duration Duration RapidRapid ResponseResponse RegulationRegulation MechanismMechanismMechanism ofofof ActionActionAction HypothalamusHypothalamusHypothalamus HypothalamicHypothalamicHypothalamic ControlControlControl PituitaryPituitaryPituitary PosteriorPosteriorPosterior PituitaryPituitaryPituitary Target Actions Oxytocin Uterus ↑ Contraction Mammary ↑ Milk let-down ADH Kidneys ↑ Water reabsorption AnteriorAnteriorAnterior PituitaryPituitaryPituitary Target Action GH Most tissue ↑ Growth TSH Thyroid ↑ TH secretion ACTH Adrenal ↑ Cortisol Cortex -

Xx250 Spc 2015 Uk
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT XENETIX 250 (250 mgI/ml) Solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION per ml 50 ml 100 ml 200 ml 500 ml Iobitridol (INN) 548.4 mg 27.42 g 54.84 g 109.68 g 274.2 g Iodine corresponding to 250 mg 12.5 g 25 g 50 g 125 g Excipient with known effect : Sodium (up to 3.5 mg per 100 mL). For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection. Clear, colourless to pale yellow solution 4. CLINICAL PARTICULARS 4.1. Therapeutic indications For adults and children undergoing: . whole-body CT . venography . intra-arterial digital subtraction angiography . ERP/ERCP This medicinal product is for diagnostic use only. 4.2. Posology and method of administration The dosage may vary depending on the type of examination, the age, weight, cardiac output and general condition of the patient and the technique used. Usually the same iodine concentration and volume are used as with other iodinated X-ray contrast in current use. As with all contrast media, the lowest dose necessary to obtain adequate visualisation should be used. Adequate hydration should be assured before and after administration as for other contrast media. As a guideline, the recommended dosages are as follows: Indications Recommended dosage Whole-body CT The doses of contrast medium and the rates of administration depend on the organs under investigation, the diagnostic problem and, in particular, the different scan and image-reconstruction times of the scanners in use. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Frequently Asked Questions for Addison Patients
FREQUENTLY ASKED QUESTIONS FOR ADDISON PATIENTS TABLE OF CONTENT (Place your cursor over the subject line, hold down the Control Button and Click your mouse. To get back to the Table of Content, hold down the Control Button and press the Home key) ADDISONIAN CRISIS / EMERGENCY ADDISONS AND OTHER DISORDERS ADDISONS AND OTHER MEDICATIONS ADRENALINE BLOOD PRESSURE COMMON COLD/FLU/OTHER ILLNESS CORTISOL DAY CURVE CORTISOL MEDICATION CRAMPS DIABETES DIAGNOSING ADDISONS EXERCISE / SPORTS FATIGUE FLORINEF / SALT / SODIUM HERBAL / VITAMIN SUPPLEMENTS HYPERPIGMENTATION IMMUNIZATION MENOPAUSE, PREGNANCY, HORMONE REPLACEMENT, BIRTH CONTROL, HYSTERECTOMY MISCELLANEOUS OSTEOPOROSIS SECONDARY ADDISON’S SHIFT WORK SLEEP 1 STRESS DOSING SURGICAL, MEDICAL, DENTAL PROCEDURES THYROID TRAVEL WEIGHT GAIN 2 ADDISONIAN CRISIS / EMERGENCY How do you know when to call an ambulance? If you are careful, you should not have to call an ambulance. If someone with adrenal insufficiency has gastrointestinal problems and is unable to keep down their cortisol or other glucocorticoid for more than 24 hrs, they should be taken to an emergency department so they can be given intravenous solucortef and saline. It is not appropriate to wait until they are so ill that they cannot be taken to the hospital by a family member. If the individual is unable to retain anything by mouth and is very ill, or if they have had a sudden stress such as a fall or an infection, then it would be necessary for them to go by ambulance as soon as possible. It is important that you should have an emergency kit at home and that someone in the household knows how to use it. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
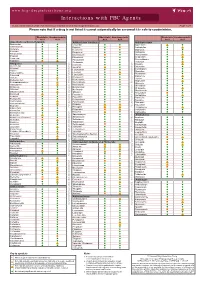
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

Guideline on Core Smpc and Package Leaflet for Gadoteric Acid EMA/CHMP/337820/2016 Page 2/23
1 26 May 2016 2 EMA/CHMP/337820/2016 3 Committee for Medicinal Products for Human Use (CHMP) 4 Guideline on core SmPC and Package Leaflet for gadoteric 5 acid 6 Draft Draft agreed by Radiopharmaceutical Drafting Group April 2016 Adopted by CHMP for release for consultation 26 May 2016 Start of public consultation 1 June 2016 End of consultation (deadline for comments) 30 September 2016 7 Comments should be provided using this template. The completed comments form should be sent to [email protected]. 8 Keywords Magnetic resonance, contrast media, gadolinium compounds, core SmPC, core Package Leaflet, gadoteric acid 9 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. 10 Guideline on core SmPC and Package Leaflet for gadoteric 11 acid 12 Table of contents 13 Executive summary ..................................................................................... 3 14 1. Introduction (background) ...................................................................... 3 15 2. Scope....................................................................................................... 3 16 3. Legal basis .............................................................................................. 3 17 4. Core SmPC and Package Leaflet for gadoteric acid ................................. -

Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Int J Clin Pharm DOI 10.1007/s11096-017-0535-2 RESEARCH ARTICLE Drug‑induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database Ying Zhao1,2,3 · Shusen Sun4 · Xiaotong Li1,3 · Xiang Ma1 · Huilin Tang5 · Lulu Sun2 · Suodi Zhai1 · Tiansheng Wang1,3,6 Received: 9 May 2017 / Accepted: 19 September 2017 © The Author(s) 2017. This article is an open access publication Abstract Background Few studies on the causes of (50.1%), mucocutaneous (47.4%), and gastrointestinal symp- drug-induced anaphylaxis (DIA) in the hospital setting are toms (31.3%). A total of 249 diferent drugs were involved. available. Objective We aimed to use the Beijing Pharma- DIAs were mainly caused by antibiotics (39.3%), traditional covigilance Database (BPD) to identify the causes of DIA Chinese medicines (TCM) (11.9%), radiocontrast agents in Beijing, China. Setting Anaphylactic case reports from (11.9%), and antineoplastic agents (10.3%). Cephalospor- the BPD provided by the Beijing Center for Adverse Drug ins accounted for majority (34.5%) of antibiotic-induced Reaction Monitoring. Method DIA cases collected by the anaphylaxis, followed by fuoroquinolones (29.6%), beta- BPD from January 2004 to December 2014 were adjudi- lactam/beta-lactamase inhibitors (15.4%) and penicillins cated. Cases were analyzed for demographics, causative (7.9%). Blood products and biological agents (3.1%), and drugs and route of administration, and clinical signs and plasma substitutes (2.1%) were also important contributors outcomes. Main outcome measure Drugs implicated in DIAs to DIAs. Conclusion A variety of drug classes were impli- were identifed and the signs and symptoms of the DIA cases cated in DIAs. -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage.