The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of Neuromuscular Blocking Effects
IndianJournalofAnesthesiaandAnalgesia OriginalResearchArticle January–February2020;7(1)(Part-I):58-63 DOI:http://dx.doi.org/10.21088/ijaa.2349.8471.7120.9 AComparativeStudyofNeuromuscularBlockingEffectsandReversibility ofCisatracuriumandVecuronium DivyaNKheskani1,HeenaSChhanwal2,BhaktiSJain3 1AssistantProfessor,2ProfessorandHead,3rdYearResident,DepartmentofAnaesthesia,GCSMedicalCollege,Hospital&Research Centre,NearChamundaBridge,Ahmedabad,Gujarat380025,India. Abstract Context: Cisatracurium is a cis isomer of parent compound atracurium, devoid of histamine release, thus possessing hemodynamic & cardiovascular stability. Aims: We compared the intubating conditions, hemodynamic stability & recovery of atracurium & vecuronium. Settings and Design: We carried out prospective, double blind randomized study after approval of ethical committee. 100 adult patients of ASA 1 &2with comparable demographicdatawereselected. Dividedin TwoGroupsC(Cisatracurium) &V(Vecuronium).Standardmonitoringwasdone&followingroutinepremedication&inductionagents, patientswereintubatedat2minsafteradministrationofcisatracurium0.15mg/kg&vecuronium0.1mg/ kgrespectively,maintainedonintermittentdoseofcisatracurium:0.03mg/kg&vecuronium:0.02mg/kg. IntubationconditionswereassessedaccordingtoTimeto25%recoveryoft1/tcfollowinginitialdoses,Time to25%recoveryoft1/tcfollowingrepeatedboluses,Timeto25%recoveryoft1/tcfollowinglastdose,Return oft4/t1ratio0.8spontaneousrecoveryatendofoperation.Statisticalanalysisused:Theresultswereevaluated byapplyingpairedt-testandp-valueusingSPSSStatisticalSoftware.Results:Intubatingconditionsat2mins -

Amidotrizoic Acid/Barium Sulfate 1477 Imbalance Should Be Corrected Before Contrast Media Are Given
Amidotrizoic Acid/Barium Sulfate 1477 imbalance should be corrected before contrast media are given. management of adhesive small bowel obstruction;2 they allow pyloric stenosis or lesions that may predispose to obstruction. Particular care is needed in patients with multiple myeloma since identification of patients who require surgery and, although they Adequate hydration should be ensured after the procedure to pre- dehydration resulting from use of contrast media may cause pre- have not been shown to relieve obstruction, they may reduce vent severe constipation. cipitation of protein in the renal tubules, leading to anuria and length of hospital stay in patients treated without surgery. It is contra-indicated in patients with gastrointestinal perforation, fatal renal failure. 1. Murshed R, et al. Meconium ileus: a ten-year review of thirty-six and should be avoided, particularly when given rectally, in those Caution is also necessary in patients with severe hypertension, patients. Eur J Pediatr Surg 1997; 7: 275–7. at risk of perforation, such as patients with acute ulcerative colitis advanced cardiac disease, phaeochromocytoma, sickle-cell dis- 2. Abbas S, et al. Oral water soluble contrast for the management or diverticulitis and after rectal or colonic biopsy, sigmoidosco- of adhesive small bowel obstruction. Available in The Cochrane ease, or hyperthyroidism or epilepsy, and in debilitated, severely Database of Systematic Reviews; Issue 3. Chichester: John Wi- py, or radiotherapy. ill, very old, or very young patients. ley; 2007 (accessed 14/07/08). Uses and Administration Amidotrizoates and other hypertonic contrast media are neuro- Preparations Barium sulfate is used as a radiographic contrast medium toxic and should not be given intrathecally; patients with sub- (p.1474) for X-ray examination of the gastrointestinal tract in- arachnoid haemorrhage may be at risk with any intravascular BP 2008: Meglumine Amidotrizoate Injection; Sodium Amidotrizoate In- jection; volving single- or double-contrast techniques or computed tom- use. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Xx250 Spc 2015 Uk
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT XENETIX 250 (250 mgI/ml) Solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION per ml 50 ml 100 ml 200 ml 500 ml Iobitridol (INN) 548.4 mg 27.42 g 54.84 g 109.68 g 274.2 g Iodine corresponding to 250 mg 12.5 g 25 g 50 g 125 g Excipient with known effect : Sodium (up to 3.5 mg per 100 mL). For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection. Clear, colourless to pale yellow solution 4. CLINICAL PARTICULARS 4.1. Therapeutic indications For adults and children undergoing: . whole-body CT . venography . intra-arterial digital subtraction angiography . ERP/ERCP This medicinal product is for diagnostic use only. 4.2. Posology and method of administration The dosage may vary depending on the type of examination, the age, weight, cardiac output and general condition of the patient and the technique used. Usually the same iodine concentration and volume are used as with other iodinated X-ray contrast in current use. As with all contrast media, the lowest dose necessary to obtain adequate visualisation should be used. Adequate hydration should be assured before and after administration as for other contrast media. As a guideline, the recommended dosages are as follows: Indications Recommended dosage Whole-body CT The doses of contrast medium and the rates of administration depend on the organs under investigation, the diagnostic problem and, in particular, the different scan and image-reconstruction times of the scanners in use. -

01012100 Pure-Bred Horses 0 0 0 0 0 01012900 Lives Horses, Except
AR BR UY Mercosu PY applied NCM Description applied applied applied r Final Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 Other bovine animals for breeding,pregnant or 01022911 lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 Other buffalo for breeding, ex. pure-bred or 01023911 pregnant 2 2 2 2 0 Other buffalo for breeding, except pure-bred 01023919 breeding 2 2 2 2 4 01023990 Other buffalos 2 2 2 2 0 01029000 Other live animals of bovine species 0 0 0 0 0 01031000 Pure-bred breedig swines 0 0 0 0 0 01039100 Other live swine, weighing less than 50 kg 2 2 2 2 0 01039200 Other live swine, weighing 50 kg or more 2 2 2 2 0 01041011 Pure-bred breeding, pregnant or lactating, sheep 0 0 0 0 0 01041019 Other pure-bred breeding sheep 0 0 0 0 0 01041090 Others live sheep 2 2 2 2 0 01042010 Pure-bred breeding goats 0 0 0 0 0 01042090 Other live goats 2 2 2 2 0 Fowls spec.gallus domestic.w<=185g pure-bred 01051110 breeding 0 0 0 0 0 Oth.live fowls spec.gall.domest.weig.not more than 01051190 185g 2 2 2 2 0 01051200 Live turkeys, weighing not more than 185g 2 2 -

Cost Analysis and Safety Comparison of Cisatracurium and Atracurium in Patients Undergoing General Anesthesia
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2013; 17: 447-450 Cost analysis and safety comparison of Cisatracurium and Atracurium in patients undergoing general anesthesia A. MOVAFEGH, S. AMINI*, H. SHARIFNIA, H. TORKAMANDI*, A. HAYATSHAHI*, M. JAVADI* Intensive Care Unit, and *Pharmaceutical Care Department; Dr. Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran Abstract. – BACKGROUND : Non-depolarizing Introduction neuromuscular blocking agents (NMB) differ in pharmacokinetic and pharmacodynamic parame - The introduction of neuromuscular blocking ters. An anesthesiologist according to these simi - larities and differences is able to choose the least agents in 1942 into anesthetic practice was an im - costly one if the same safety profile and same clin - portant development. Non-depolarizing NMB ical benefit achieved with the different alternatives. agents differ in the onset of action, duration of ac - AIM: The main objective of this study is to eval - tion, metabolic route, potency, adverse effects and uate the economic and adverse drug reactions cost. An anesthesiologist is able to choose NMB prevalence and differences between cisatracurium drugs according to these similarities and differ - and atracurium the two non-depolarizing NMB 1,2 drugs, which are widely used in adult patients un - ences . Atracurium and Cisatracurium are two dergoing surgery with general anesthesia in a non-depolarizing NMB agents with intermediate teaching Hospital in Iran. duration of action 1. Cisatracurium besilate is the MATERIALS AND METHODS : A cost analysis R-cis isomer of atracurium besilate and is 3-4 fold and adverse drug reactions (ADR) monitoring more potent than atracurium 3. Compared with were performed. -

Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Int J Clin Pharm DOI 10.1007/s11096-017-0535-2 RESEARCH ARTICLE Drug‑induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database Ying Zhao1,2,3 · Shusen Sun4 · Xiaotong Li1,3 · Xiang Ma1 · Huilin Tang5 · Lulu Sun2 · Suodi Zhai1 · Tiansheng Wang1,3,6 Received: 9 May 2017 / Accepted: 19 September 2017 © The Author(s) 2017. This article is an open access publication Abstract Background Few studies on the causes of (50.1%), mucocutaneous (47.4%), and gastrointestinal symp- drug-induced anaphylaxis (DIA) in the hospital setting are toms (31.3%). A total of 249 diferent drugs were involved. available. Objective We aimed to use the Beijing Pharma- DIAs were mainly caused by antibiotics (39.3%), traditional covigilance Database (BPD) to identify the causes of DIA Chinese medicines (TCM) (11.9%), radiocontrast agents in Beijing, China. Setting Anaphylactic case reports from (11.9%), and antineoplastic agents (10.3%). Cephalospor- the BPD provided by the Beijing Center for Adverse Drug ins accounted for majority (34.5%) of antibiotic-induced Reaction Monitoring. Method DIA cases collected by the anaphylaxis, followed by fuoroquinolones (29.6%), beta- BPD from January 2004 to December 2014 were adjudi- lactam/beta-lactamase inhibitors (15.4%) and penicillins cated. Cases were analyzed for demographics, causative (7.9%). Blood products and biological agents (3.1%), and drugs and route of administration, and clinical signs and plasma substitutes (2.1%) were also important contributors outcomes. Main outcome measure Drugs implicated in DIAs to DIAs. Conclusion A variety of drug classes were impli- were identifed and the signs and symptoms of the DIA cases cated in DIAs. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Antioxidant, Anti-Inflammatory, Anti-Radiation, Metal Chelating Compounds and Uses Thereof
(19) TZZ¥_ ___T (11) EP 3 121 189 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 25.01.2017 Bulletin 2017/04 C07K 5/023 (2006.01) A61K 38/07 (2006.01) A61P 17/00 (2006.01) A61P 25/00 (2006.01) (21) Application number: 16162206.3 (22) Date of filing: 19.01.2012 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • MOGRABI, Josef GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 69379 Tel Aviv (IL) PL PT RO RS SE SI SK SM TR • ATLAS, Daphne 93714 Jerusalem (IL) (30) Priority: 20.01.2011 US 201161434454 P • KEYNAN, Shoshana 30.03.2011 US 201161469138 P 71702 Modiine (IL) (62) Document number(s) of the earlier application(s) in (74) Representative: Becker Kurig Straus accordance with Art. 76 EPC: Patentanwälte 12705459.1 / 2 665 742 Bavariastrasse 7 80336 München (DE) (27) Previously filed application: 19.01.2012 PCT/IL2012/000032 Remarks: This application was filed on 24-03-2016 as a (71) Applicants: divisional application to the application mentioned • Oneday - Biotech And Pharma Ltd. under INID code 62. 6789717 Tel Aviv (IL) • Yissum Research Development Company of the Hebrew University of Jerusalem Ltd. Jerusalem 91390 (IL) (54) ANTIOXIDANT, ANTI-INFLAMMATORY, ANTI-RADIATION, METAL CHELATING COMPOUNDS AND USES THEREOF (57) The present invention relates to potent compounds having combined antioxidant, antiinflammatory, anti-radiation and metal chelating properties. Specifically, the present invention relates to short peptides having said properties, and to methods and uses of such short peptides in clinical and cosmetic applications. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -
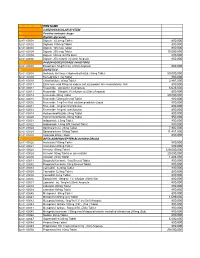
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Page 1 Note: Within Nine Months from the Publication of the Mention
Europäisches Patentamt (19) European Patent Office & Office européen des brevets (11) EP 1 411 992 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 49/04 (2006.01) A61K 49/18 (2006.01) 13.12.2006 Bulletin 2006/50 (86) International application number: (21) Application number: 02758379.8 PCT/EP2002/008183 (22) Date of filing: 23.07.2002 (87) International publication number: WO 2003/013616 (20.02.2003 Gazette 2003/08) (54) IONIC AND NON-IONIC RADIOGRAPHIC CONTRAST AGENTS FOR USE IN COMBINED X-RAY AND NUCLEAR MAGNETIC RESONANCE DIAGNOSTICS IONISCHES UND NICHT-IONISCHES RADIOGRAPHISCHES KONTRASTMITTEL ZUR VERWENDUNG IN DER KOMBINIERTEN ROENTGEN- UND KERNSPINTOMOGRAPHIEDIAGNOSTIK SUBSTANCES IONIQUES ET NON-IONIQUES DE CONTRASTE RADIOGRAPHIQUE UTILISEES POUR ETABLIR DES DIAGNOSTICS FAISANT APPEL AUX RAYONS X ET A L’IMAGERIE PAR RESONANCE MAGNETIQUE (84) Designated Contracting States: (74) Representative: Minoja, Fabrizio AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Bianchetti Bracco Minoja S.r.l. IE IT LI LU MC NL PT SE SK TR Via Plinio, 63 20129 Milano (IT) (30) Priority: 03.08.2001 IT MI20011706 (56) References cited: (43) Date of publication of application: EP-A- 0 759 785 WO-A-00/75141 28.04.2004 Bulletin 2004/18 US-A- 5 648 536 (73) Proprietor: BRACCO IMAGING S.p.A. • K HERGAN, W. DORINGER, M. LÄNGLE W.OSER: 20134 Milano (IT) "Effects of iodinated contrast agents in MR imaging" EUROPEAN JOURNAL OF (72) Inventors: RADIOLOGY, vol. 21, 1995, pages 11-17, • AIME, Silvio XP002227102 20134 Milano (IT) • K.M.