Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Country Reports Japan International Corporation of Welfare Services (JICWELS) Contents 1. Cambodia 1 2. Indonesia 70 3. Malaysia 91 4. Philippines 116 5. Sri Lanka 141 6. Thailand 161 The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Cambodia -1- KINGDOM OF CAMBODIA Nation Religion King Ministry of Health Department of Drugs and Food Country Report The Study Program on Quality Management of Essential Medicines Good Manufacturing Practice (GMP) and Inspection November 4, 2012 – November 30, 2012 Sponsored by : The Government of Japan Japan International Cooperation Agency (JICA) Department of Drugs and Food Ministry of Health, Cambodia. -2- I- COUNTRY PROFILE -3- A-Geography Cambodia is an agricultural country located in South East Asia which bordering the Gulf of Thailand, between Thailand, Vietnam, and Laos. Its approximate geographical coordinates are 13°N 105°E. Its 2,572 km border is split among Vietnam (1,228 km), Thailand (803 km) and Laos (541 km), as well as 443 km of coastline. Cambodia covers 181,035 square kilometers in the southwestern part of the Indochina, Cambodia lies completely within the tropics; its southernmost points are only slightly more than 10° above the equator. The country is bounded on the north by Thailand and by Laos, on the east and southeast by Vietnam, and on the west by the Gulf of Thailand and by Thailand. It consists of the Tonle Sap Basin and the Mekong Lowlands. To the southeast of this great basin is the Mekong Delta, which extends through Vietnam to the South China Sea. -

Download Download
VOLUME 7 NOMOR 2 DESEMBER 2020 ISSN 2548 – 611X JURNAL BIOTEKNOLOGI & BIOSAINS INDONESIA Homepage Jurnal: http://ejurnal.bppt.go.id/index.php/JBBI IN SILICO STUDY OF CEPHALOSPORIN DERIVATIVES TO INHIBIT THE ACTIONS OF Pseudomonas aeruginosa Studi In Silico Senyawa Turunan Sefalosporin dalam Menghambat Aktivitas Bakteri Pseudomonas aeruginosa Saly Amaliacahya Aprilian*, Firdayani, Susi Kusumaningrum Pusat Teknologi Farmasi dan Medika, BPPT, Gedung LAPTIAB 610-612 Kawasan Puspiptek, Setu, Tangerang Selatan, Banten 15314 *Email: [email protected] ABSTRAK Infeksi yang diakibatkan oleh bakteri gram-negatif, seperti Pseudomonas aeruginosa telah menyebar luas di seluruh dunia. Hal ini menjadi ancaman terhadap kesehatan masyarakat karena merupakan bakteri yang multi-drug resistance dan sulit diobati. Oleh karena itu, pentingnya pengembangan agen antimikroba untuk mengobati infeksi semakin meningkat dan salah satu yang saat ini banyak dikembangkan adalah senyawa turunan sefalosporin. Penelitian ini melakukan studi mengenai interaksi tiga dimensi (3D) antara antibiotik dari senyawa turunan Sefalosporin dengan penicillin-binding proteins (PBPs) pada P. aeruginosa. Tujuan dari penelitian ini adalah untuk mengklarifikasi bahwa agen antimikroba yang berasal dari senyawa turunan sefalosporin efektif untuk menghambat aktivitas bakteri P. aeruginosa. Struktur PBPs didapatkan dari Protein Data Bank (PDB ID: 5DF9). Sketsa struktur turunan sefalosporin digambar menggunakan Marvins Sketch. Kemudian, studi mengenai interaksi antara antibiotik dan PBPs dilakukan menggunakan program Mollegro Virtual Docker 6.0. Hasil yang didapatkan yaitu nilai rerank score terendah dari kelima generasi sefalosporin, di antaranya sefalotin (-116.306), sefotetan (-133.605), sefoperazon (-160.805), sefpirom (- 144.045), dan seftarolin fosamil (-146.398). Keywords: antibiotik, penicillin-binding proteins, P. aeruginosa, sefalosporin, studi interaksi ABSTRACT Infections caused by gram-negative bacteria, such as Pseudomonas aeruginosa, have been spreading worldwide. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Sulfur Hexafluoride Hazard Summary Identification
Common Name: SULFUR HEXAFLUORIDE CAS Number: 2551-62-4 RTK Substance number: 1760 DOT Number: UN 1080 Date: April 2002 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Sulfur Hexafluoride can affect you when breathed in. * If you think you are experiencing any work-related health * Sulfur Hexafluoride can irritate the skin causing a rash or problems, see a doctor trained to recognize occupational burning feeling on contact. Direct skin contact can cause diseases. Take this Fact Sheet with you. frostbite. * Exposure to hazardous substances should be routinely * Sulfur Hexafluoride may cause severe eye burns leading evaluated. This may include collecting personal and area to permanent damage. air samples. You can obtain copies of sampling results * Breathing Sulfur Hexafluoride can irritate the nose and from your employer. You have a legal right to this throat. information under OSHA 1910.1020. * Breathing Sulfur Hexafluoride may irritate the lungs causing coughing and/or shortness of breath. Higher WORKPLACE EXPOSURE LIMITS exposures can cause a build-up of fluid in the lungs OSHA: The legal airborne permissible exposure limit (pulmonary edema), a medical emergency, with severe (PEL) is 1,000 ppm averaged over an 8-hour shortness of breath. workshift. * High exposure can cause headache, confusion, dizziness, suffocation, fainting, seizures and coma. NIOSH: The recommended airborne exposure limit is * Sulfur Hexafluoride may damage the liver and kidneys. 1,000 ppm averaged over a 10-hour workshift. * Repeated high exposure can cause deposits of Fluorides in the bones and teeth, a condition called “Fluorosis.” This ACGIH: The recommended airborne exposure limit is may cause pain, disability and mottling of the teeth. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Imaging Diagnostics by Combining Contrast Agents
(19) TZZ Z _T (11) EP 2 907 526 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.08.2015 Bulletin 2015/34 A61K 49/04 (2006.01) A61K 51/04 (2006.01) A61K 49/06 (2006.01) (21) Application number: 15155866.5 (22) Date of filing: 20.06.2008 (84) Designated Contracting States: (72) Inventor: Wiebelitz, Ulrike AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 13158 Berlin (DE) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR (74) Representative: Kilger, Ute Boehmert & Boehmert (30) Priority: 22.06.2007 EP 07110922 Anwaltspartnerschaft mbB Patentanwälte Rechtsanwälte (62) Document number(s) of the earlier application(s) in Pettenkoferstrasse 20-22 accordance with Art. 76 EPC: 80336 München (DE) 12199299.4 / 2 572 735 12168380.9 / 2 514 442 Remarks: 08774183.1 / 2 170 405 This application was filed on 20-02-2015 as a divisional application to the application mentioned (71) Applicant: mivenion GmbH under INID code 62. 10115 Berlin (DE) (54) Imaging diagnostics by combining contrast agents (57) The present invention relates to the use of a combination of several contrast agents having different imaging properties with respect to imaging representation. EP 2 907 526 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 2 907 526 A1 2 Description from suspect lesions and to prepare and assess these samples histopathologically. [0001] The present invention relates to the use of a [0005] MRI examination of the female breast has very combination of several contrast agents having different high sensitivity in comparison to other imaging modali- imaging properties. -

Guideline on Core Smpc and Package Leaflet for Gadoteric Acid EMA/CHMP/337820/2016 Page 2/23
1 26 May 2016 2 EMA/CHMP/337820/2016 3 Committee for Medicinal Products for Human Use (CHMP) 4 Guideline on core SmPC and Package Leaflet for gadoteric 5 acid 6 Draft Draft agreed by Radiopharmaceutical Drafting Group April 2016 Adopted by CHMP for release for consultation 26 May 2016 Start of public consultation 1 June 2016 End of consultation (deadline for comments) 30 September 2016 7 Comments should be provided using this template. The completed comments form should be sent to [email protected]. 8 Keywords Magnetic resonance, contrast media, gadolinium compounds, core SmPC, core Package Leaflet, gadoteric acid 9 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. 10 Guideline on core SmPC and Package Leaflet for gadoteric 11 acid 12 Table of contents 13 Executive summary ..................................................................................... 3 14 1. Introduction (background) ...................................................................... 3 15 2. Scope....................................................................................................... 3 16 3. Legal basis .............................................................................................. 3 17 4. Core SmPC and Package Leaflet for gadoteric acid ................................. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -
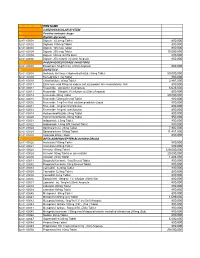
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Page 1 Note: Within Nine Months from the Publication of the Mention
Europäisches Patentamt (19) European Patent Office & Office européen des brevets (11) EP 1 411 992 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 49/04 (2006.01) A61K 49/18 (2006.01) 13.12.2006 Bulletin 2006/50 (86) International application number: (21) Application number: 02758379.8 PCT/EP2002/008183 (22) Date of filing: 23.07.2002 (87) International publication number: WO 2003/013616 (20.02.2003 Gazette 2003/08) (54) IONIC AND NON-IONIC RADIOGRAPHIC CONTRAST AGENTS FOR USE IN COMBINED X-RAY AND NUCLEAR MAGNETIC RESONANCE DIAGNOSTICS IONISCHES UND NICHT-IONISCHES RADIOGRAPHISCHES KONTRASTMITTEL ZUR VERWENDUNG IN DER KOMBINIERTEN ROENTGEN- UND KERNSPINTOMOGRAPHIEDIAGNOSTIK SUBSTANCES IONIQUES ET NON-IONIQUES DE CONTRASTE RADIOGRAPHIQUE UTILISEES POUR ETABLIR DES DIAGNOSTICS FAISANT APPEL AUX RAYONS X ET A L’IMAGERIE PAR RESONANCE MAGNETIQUE (84) Designated Contracting States: (74) Representative: Minoja, Fabrizio AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Bianchetti Bracco Minoja S.r.l. IE IT LI LU MC NL PT SE SK TR Via Plinio, 63 20129 Milano (IT) (30) Priority: 03.08.2001 IT MI20011706 (56) References cited: (43) Date of publication of application: EP-A- 0 759 785 WO-A-00/75141 28.04.2004 Bulletin 2004/18 US-A- 5 648 536 (73) Proprietor: BRACCO IMAGING S.p.A. • K HERGAN, W. DORINGER, M. LÄNGLE W.OSER: 20134 Milano (IT) "Effects of iodinated contrast agents in MR imaging" EUROPEAN JOURNAL OF (72) Inventors: RADIOLOGY, vol. 21, 1995, pages 11-17, • AIME, Silvio XP002227102 20134 Milano (IT) • K.M. -

Consideration of Antibacterial Medicines As Part Of
Consideration of antibacterial medicines as part of the revisions to 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) Section 6.2 Antibacterials including Access, Watch and Reserve Lists of antibiotics This summary has been prepared by the Health Technologies and Pharmaceuticals (HTP) programme at the WHO Regional Office for Europe. It is intended to communicate changes to the 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) to national counterparts involved in the evidence-based selection of medicines for inclusion in national essential medicines lists (NEMLs), lists of medicines for inclusion in reimbursement programs, and medicine formularies for use in primary, secondary and tertiary care. This document does not replace the full report of the WHO Expert Committee on Selection and Use of Essential Medicines (see The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2019 (WHO Technical Report Series, No. 1021). Licence: CC BY-NC-SA 3.0 IGO: https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1) and Corrigenda (March 2020) – TRS1021 (https://www.who.int/medicines/publications/essentialmedicines/TRS1021_corrigenda_March2020. pdf?ua=1). Executive summary of the report: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO- MVP-EMP-IAU-2019.05-eng.pdf?ua=1.