Estramustine Sodium Phosphate Capsules BP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2005/0070488A1 Coelingh Bennik Et Al
US 2005.0070488A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0070488A1 Coelingh Bennik et al. (43) Pub. Date: Mar. 31, 2005 (54) ESTROGENIC COMPOUNDS IN prises the oral administration of an estrogenic component COMBINATION WITH PROGESTOGENIC and a progestogenic component to a mammal in an effective COMPOUNDS IN amount to prevent or treat Symptoms of hypoestrogenism, HORMONE-REPLACEMENT THERAPY wherein the estrogenic component is Selected from the group (76) Inventors: Herman Jan Tijmen Coelingh Bennik, consisting of Substances represented by the above formula in Driebergen (NL); Evert Johannes which formula R, R2, R, R independently are a hydrogen Bunschoten, Heesch (NL); Christian atom, a hydroxyl group or an alkoxy group with 1-5 carbon Franz Holinka, New York, NY (US) atoms; each of Rs, R, R-7 is a hydroxyl group; and no more than 3 of R, R2, R, R are hydrogen atoms; precursors Correspondence Address: capable of liberating a Substance according to the aforemen William Logsdon tioned formula when used in the present method; and Webb Ziesenheim Logsdon Orkin & Hanson mixtures of one or more of the aforementioned Substances 700 Koppers Building and/or precursors. Another aspect of the invention concerns 436 Seventh Avenue a pharmaceutical kit comprising oral dosage units that Pittsburgh, PA 15219-1818 (US) contain the aforementioned estrogenic component and a (21) Appl. No.: 10/495,707 progestogenic component as well as an androgenic compo nent. (22) PCT Filed: May 23, 2002 (86) PCT No.: PCT/NL02/00332 (30) Foreign Application Priority Data Nov. 15, 2001 (EP)........................................ O1204377.4 Feb. -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

PLGG1, a Plastidic Glycolate Glycerate Transporter, Is Required for Photorespiration and Defines a Unique Class of Metabolite Transporters
PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters Thea R. Picka,1, Andrea Bräutigama,1, Matthias A. Schulza, Toshihiro Obatab, Alisdair R. Fernieb, and Andreas P. M. Webera,2 aInstitute of Plant Biochemistry, Cluster of Excellence on Plant Sciences, Heinrich Heine University, 40225 Düsseldorf, Germany; and bMax-Planck Institute for Molecular Plant Physiology, Department of Molecular Physiology, 14476 Potsdam-Golm, Germany Edited by Wolf B. Frommer, Carnegie Institution for Science, Stanford, CA, and accepted by the Editorial Board January 8, 2013 (received for review September 4, 2012) Photorespiratory carbon flux reaches up to a third of photosyn- (PGLP). Glycolate is exported from the chloroplasts to the per- thetic flux, thus contributes massively to the global carbon cycle. oxisomes, where it is oxidized to glyoxylate by glycolate oxidase The pathway recycles glycolate-2-phosphate, the most abundant (GOX) and transaminated to glycine by Ser:glyoxylate and Glu: byproduct of RubisCO reactions. This oxygenation reaction of glyoxylate aminotransferase (SGT and GGT, respectively). Glycine RubisCO and subsequent photorespiration significantly limit the leaves the peroxisomes and enters the mitochondria, where two biomass gains of many crop plants. Although photorespiration is molecules of glycine are deaminated and decarboxylated by the a compartmentalized process with enzymatic reactions in the glycine decarboxylase complex (GDC) and serine hydroxymethyl- chloroplast, the peroxisomes, the mitochondria, and the cytosol, transferase (SHMT) to form one molecule each of serine, ammo- nia, and carbon dioxide. Serine is exported from the mitochondria no transporter required for the core photorespiratory cycle has to the peroxisomes, where it is predominantly converted to glyc- been identified at the molecular level to date. -

The Self-Inhibitory Nature of Metabolic Networks and Its Alleviation Through Compartmentalization
ARTICLE Received 30 Oct 2016 | Accepted 23 May 2017 | Published 10 Jul 2017 DOI: 10.1038/ncomms16018 OPEN The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization Mohammad Tauqeer Alam1,2, Viridiana Olin-Sandoval1,3, Anna Stincone1,w, Markus A. Keller1,4, Aleksej Zelezniak1,5,6, Ben F. Luisi1 & Markus Ralser1,5 Metabolites can inhibit the enzymes that generate them. To explore the general nature of metabolic self-inhibition, we surveyed enzymological data accrued from a century of experimentation and generated a genome-scale enzyme-inhibition network. Enzyme inhibition is often driven by essential metabolites, affects the majority of biochemical processes, and is executed by a structured network whose topological organization is reflecting chemical similarities that exist between metabolites. Most inhibitory interactions are competitive, emerge in the close neighbourhood of the inhibited enzymes, and result from structural similarities between substrate and inhibitors. Structural constraints also explain one-third of allosteric inhibitors, a finding rationalized by crystallographic analysis of allosterically inhibited L-lactate dehydrogenase. Our findings suggest that the primary cause of metabolic enzyme inhibition is not the evolution of regulatory metabolite–enzyme interactions, but a finite structural diversity prevalent within the metabolome. In eukaryotes, compartmentalization minimizes inevitable enzyme inhibition and alleviates constraints that self-inhibition places on metabolism. 1 Department of Biochemistry and Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK. 2 Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. 3 Department of Food Science and Technology, Instituto Nacional de Ciencias Me´dicas y Nutricio´n Salvador Zubira´n, Vasco de Quiroga 15, Tlalpan, 14080 Mexico City, Mexico. -

Journal Pre-Proof
Journal Pre-proof Steroid hormones in the aquatic environment J.O. Ojoghoro, M.D. Scrimshaw, J.P. Sumpter PII: S0048-9697(21)03377-5 DOI: https://doi.org/10.1016/j.scitotenv.2021.148306 Reference: STOTEN 148306 To appear in: Science of the Total Environment Received date: 19 April 2021 Revised date: 3 June 2021 Accepted date: 3 June 2021 Please cite this article as: J.O. Ojoghoro, M.D. Scrimshaw and J.P. Sumpter, Steroid hormones in the aquatic environment, Science of the Total Environment (2018), https://doi.org/10.1016/j.scitotenv.2021.148306 This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. © 2018 © 2021 Published by Elsevier B.V. Journal Pre-proof Steroid Hormones in the Aquatic Environment Ojoghoro, J.O.a, Scrimshaw, M.D.b* and Sumpter, J.P.b a Department of Botany, Faculty of Science, Delta State University Abraka, Delta State, Nigeria b Division of Environmental Science, Department of Life Sciences, Brunel University London, Uxbridge, Middlesex, UB8 3PH, United Kingdom Abstract Steroid hormones are extremely important natural hormones in all vertebrates. -

Fee-For-Service Preferred Drug List
Prescription Drug Program Apple Health Medicaid: Fee-for-Service Preferred Drug List What is new in this version of the preferred drug list? Effective for dates of service on and after October 1, 2018, the Health Care Authority will make the following changes: Change Due to the implementation of the Apple Health Preferred Drug List (PDL), a PDL that applies to fee-for-service (FFS) clients as well as Apple Health managed care enrollees, the following changes have occurred: On the Fee-For-Service only Preferred Drug List • Drug classes that are currently on the Apple Health PDL have been removed. These classes and the drug statuses can be found on the Apple Health Preferred Drug List. On the Apple Health Preferred Drug List • New drug classes have been added. This means drugs not previously on the PDL have been added with preferred and nonpreferred statuses. Some drugs may also have additional prior authorization (PA) requirements. • For existing drug classes, preferred statuses may have changed. Some drugs may have additional PA requirements that did not previously require PA. October 25, 2018 Correction • Two drug classes were erroneously removed and have been added back to the list: Asthma – Leukotriene Modifiers Skeletal Muscle Relaxants (Rev. 10/24/2018) (Eff. 10/1/2018) – 1 – Apple Health Medicaid PDL Prescription Drug Program What is the preferred drug list? The Health Care Authority (the agency) has developed a list of preferred drugs within a chosen therapeutic class that are selected based on clinical evidence of safety, efficacy, and effectiveness. The drugs within a chosen therapeutic class are evaluated by the Drug Use Review Board, which makes recommendations to the agency regarding the selection of the preferred drugs. -
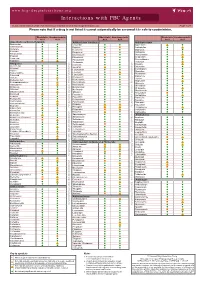
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Confidential: for Review Only
BMJ Confidential: For Review Only The risk of fall and fracture with the initiation of a prostate - selective alpha antagonist Journal: BMJ Manuscript ID: BMJ.2015.028205 Article Type: Research BMJ Journal: BMJ Date Submitted by the Author: 17-Jul-2015 Complete List of Authors: Welk, Blayne; Western University, McArthur, Eric; Institute for Clinical Evaluative Sciences,, Fraser, Lisa-Ann; Western University, Medicine Hayward, Jade; Institute for Clinical Evaluative Sciences,, Dixon, Stephanie; Institute for Clinical Evaluative Sciences,, Hwang, Joseph; Case Western Reserve University School of Medicine, Ordon, Michael; University of Toronto, Surgery (Urology) Keywords: BPH, Fall, Fracture, Alpha antgonist https://mc.manuscriptcentral.com/bmj Page 1 of 45 BMJ 1 2 The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist 3 4 1,2,3 2 4 2 5 Blayne Welk MD MSc , Eric McArthur MSc , Lisa-Ann Fraser MD MSc , Jade Hayward , 6 Stephanie Dixon MSc PhD 2,3 , Y. Joseph Hwang MSc 5, Michael Ordon MD MSc 6 7 8 1 DepartmentConfidential: of Surgery, Western University, For London Review, Ontario, Canada Only 9 2 Institute for Clinical Evaluative Sciences, London, Ontario, Canada 10 11 3 Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada 12 4 Department of Medicine, Western University, London, Ontario 13 5 MD Candidate, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA 14 6 Division of Urology, Department of Surgery, University of Toronto, Toronto, Ontario, Canada 15 16 17 Correspondence: 18 Blayne Welk, MD MSc 19 Assistant Professor, Division of Urology and Epidemiology and Biostatistics 20 Western University 21 Room B4-667 22 23 St Joseph's Health Care 24 268 Grosvenor Street London ON N6A 4V2 25 Telephone: 519 646-6367 | Fax: 519 646-6037 26 [email protected] 27 28 29 Addresses: 30 Mr McArthur: [email protected] 31 LHSC – Victoria Hospital 32 ELL-101, 800 Commissioners Rd. -

Progesterone 7K77 49-3265/R3 B7K770 Read Highlighted Changes Revised April, 2010 Progesterone
en system Progesterone 7K77 49-3265/R3 B7K770 Read Highlighted Changes Revised April, 2010 Progesterone Customer Service: Contact your local representative or find country specific contact information on www.abbottdiagnostics.com Package insert instructions must be carefully followed. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert. Key to symbols used List Number Calibrator (1,2) In Vitro Diagnostic Medical Control Low, Medium, High Device (L, M, H) Lot Number Reagent Lot Expiration Date Reaction Vessels Serial Number Sample Cups Septum Store at 2-8°C Replacement Caps Consult instructions for use Warning: May cause an allergic reaction Contains sodium azide. Contact Manufacturer with acids liberates very toxic gas. See REAGENTS section for a full explanation of symbols used in reagent component naming. 1 NAME REAGENTS ARCHITECT Progesterone Reagent Kit, 100 Tests INTENDED USE NOTE: Some kit sizes are not available in all countries or for use on all ARCHITECT i Systems. Please contact your local distributor. The ARCHITECT Progesterone assay is a Chemiluminescent Microparticle Immunoassay (CMIA) for the quantitative determination of progesterone in ARCHITECT Progesterone Reagent Kit (7K77) human serum and plasma. • 1 or 4 Bottle(s) (6.6 mL) Anti-fluorescein (mouse, monoclonal) fluorescein progesterone complex coated Microparticles SUMMARY AND EXPLANATION OF TEST in TRIS buffer with protein (bovine and murine) and surfactant Progesterone is produced primarily by the corpus luteum of the ovary stabilizers. Concentration: 0.1% solids. Preservatives: sodium azide in normally menstruating women and to a lesser extent by the adrenal and ProClin. cortex.1 At approximately the 6th week of pregnancy, the placenta 2-5 • 1 or 4 Bottle(s) (17.0 mL) Anti-progesterone (sheep, becomes the major producer of progesterone. -

Resistance to Antifolates
Oncogene (2003) 22, 7431–7457 & 2003 Nature Publishing Group All rights reserved 0950-9232/03 $25.00 www.nature.com/onc Resistance to antifolates Rongbao Zhao1 and I David Goldman*,1 1Departments of Medicine and Molecular, Pharmacology, Albert Einstein College of Medicine, Bronx, New York, USA The antifolates were the first class of antimetabolites to the kinetics of the interaction between MTX and DHFR enter the clinics more than 50 years ago. Over the was fully understood, and not until the late 1970s and following decades, a full understanding of their mechan- early 1980s when polyglutamate derivatives of MTX were isms of action and chemotherapeutic potential evolved detected and their pharmacologic importance clarified. along with the mechanisms by which cells develop Likewise, an understanding of tumor cell resistance to resistance to these drugs. These principals served as a antifolates evolved slowly, often paralleling the emergence basis for the subsequent exploration and understanding of of new molecular concepts. As the mechanisms of the mechanisms of resistance to a variety of diverse resistance to antifolates were characterized, this provided antineoplastics with different cellular targets. This section insights and principles that were broadly applicable to describes the bases for intrinsic and acquired antifolate other antineoplastics. Ultimately, this knowledge led to the resistance within the context of the current understanding development of a new generation of antifolates, in the late of the mechanisms of actions and cytotoxic determinants 1980s and 1990s, which are potent direct inhibitors of of these agents. This encompasses impaired drug transport tetrahydrofolate (THF)-cofactor-dependent enzymes. Sev- into cells, augmented drug export, impaired activation of eral of these drugs are now in clinical trials, and the antifolates through polyglutamylation, augmented hydro- activity of one, pemetrexed, has been confirmed in a large lysis of antifolate polyglutamates, increased expression Phase III trial (Vogelzang et al., 2003).