PDF Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jarvis Island NWR Final
Jarvis Island National Wildlife Refuge Comprehensive Conservation Plan FINDING OF NO SIGNIFICANT IMPACT Jarvis Island National Wildlife Refuge Comprehensive Conservation Plan Unincorporated U.S. Territory, Central Pacific Ocean The U.S. Fish and Wildlife Service (Service) has completed the Comprehensive Conservation Plan (CCP) and Environmental Assessment (EA) for Jarvis Island National Wildlife Refuge (Refuge). The CCP will guide management of the Refuge for the next 15 years. The CCP and EA describe the Service’s preferred alternative for managing the Refuge and its effects on the human environment. Decision Following comprehensive review and analysis, the Service selected Alternative B in the draft EA for implementation because it is the alternative that best meets the following criteria: Achieves the mission of the National Wildlife Refuge System. Achieves the purposes of the Refuge. Will be able to achieve the vision and goals for the Refuge. Maintains and restores the ecological integrity of the habitats and plant and animal populations at the Refuge. Addresses the important issues identified during the scoping process. Addresses the legal mandates of the Service and the Refuge. Is consistent with the scientific principles of sound wildlife management. Can be implemented within the projected fiscal and logistical management constraints associated with the Refuge’s remote location. As described in detail in the CCP and EA, implementing the selected alternative will have no significant impacts on any of the natural or cultural resources identified in the CCP and EA. Public Review The planning process incorporated a variety of public involvement techniques in developing and reviewing the CCP. This included three planning updates, meetings with partners, and public review and comment on the planning documents. -

Reef Fishes of the Bird's Head Peninsula, West
Check List 5(3): 587–628, 2009. ISSN: 1809-127X LISTS OF SPECIES Reef fishes of the Bird’s Head Peninsula, West Papua, Indonesia Gerald R. Allen 1 Mark V. Erdmann 2 1 Department of Aquatic Zoology, Western Australian Museum. Locked Bag 49, Welshpool DC, Perth, Western Australia 6986. E-mail: [email protected] 2 Conservation International Indonesia Marine Program. Jl. Dr. Muwardi No. 17, Renon, Denpasar 80235 Indonesia. Abstract A checklist of shallow (to 60 m depth) reef fishes is provided for the Bird’s Head Peninsula region of West Papua, Indonesia. The area, which occupies the extreme western end of New Guinea, contains the world’s most diverse assemblage of coral reef fishes. The current checklist, which includes both historical records and recent survey results, includes 1,511 species in 451 genera and 111 families. Respective species totals for the three main coral reef areas – Raja Ampat Islands, Fakfak-Kaimana coast, and Cenderawasih Bay – are 1320, 995, and 877. In addition to its extraordinary species diversity, the region exhibits a remarkable level of endemism considering its relatively small area. A total of 26 species in 14 families are currently considered to be confined to the region. Introduction and finally a complex geologic past highlighted The region consisting of eastern Indonesia, East by shifting island arcs, oceanic plate collisions, Timor, Sabah, Philippines, Papua New Guinea, and widely fluctuating sea levels (Polhemus and the Solomon Islands is the global centre of 2007). reef fish diversity (Allen 2008). Approximately 2,460 species or 60 percent of the entire reef fish The Bird’s Head Peninsula and surrounding fauna of the Indo-West Pacific inhabits this waters has attracted the attention of naturalists and region, which is commonly referred to as the scientists ever since it was first visited by Coral Triangle (CT). -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

LABRIDAE Wrasses Are Variable in Shape, Coloration
click for previous page LABR 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) LABRIDAE Wrasses, hogfishes, razorfishes, coris, tuskfishes Wrasses are variable in shape, coloration and size, though the majority of the species attain a maximum length of less than 20 cm. Body slightly to extremely compressed. Mouth terminal, usually with prominent lips; jaws slightly to extremely protrusible; teeth in jaws usually separate and caniniform, the anteriormost 1 or 2 pairs typically enlarged and directed somewhat forward; a few forms with lateral teeth reduced and coalesced to form a bony cutting edge and anteriormost teeth modified into prominent incisors; pharyngeal teeth (located at base of throat) strong. A single, long-based dorsal fin, although in some species (e.g., Xyrichthys) the first 2 spines are more or less removed from the rest of fin; spines pungent to flexible; spines and soft rays usually of similar length, though the first few spines or certain soft rays may be elongate in some. Scales cycloid (smooth to touch), usually of moderate to large size, although very small in some forms; lateral lines smoothly curved or with an abrupt curve below soft portion of dorsal fin, either continuous or interrupted below posterior part of dorsal fin. Colour: generally bright and elaborately patterened, often differing between sexes and changing with age. Xyrichtys Cheilinus Thalassoma labrids of different shape Cheilio - 2 - FAO Sheets LABRIDAE Fishing Area 51 Common in shallow to moderately shallow wates in a variety of habitats, including bare sand and rock, grass and algae-covered bottoms and coral reefs, but rare in muddy areas. -

Chumbe Island Coral Park Conservation and Education Status Report 2013
Chumbe Island Coral Park Conservation and Education Status Report 2013 Zanzibar, Tanzania Index Foreword………………………………………………………………………………… 3 Part II: Environmental Education……………………………………………………... 25 Introduction CHICOP…………………………………………………………………... 4 Management Plan 2006-2016…………………………………………………… 26 Chumbe Field Excursions………………………………………………………… 27 Part I: Conservation Programs………………………………………………………. 5 Educational Outcomes……………………………………………………………. 28 Management Plan 2006 – 2016…………………………………………………. 6 The Chumbe Challenge………………………………………………………….. 29 Key Values of the MPA…………………………………………………………… 7 Community Outreach …………………………………………………………….. 30 Chumbe Reef Sanctuary (CRS) ………………………………………………… 8 Island Ranger Training……………………………………………………………. 31 Borders of the CRS ………………………………………………………………. 9 Chumbe aims Zero Waste………………………………………………………... 32 Tresspassing ……………………………………………………………………… 10 Celebration of International Events……………………………………………… 33 Fauna in the CRS…………………………………………………………………. 11 Monitoring Programs……………………………………………………………… 12 Acknowledgements……………………………………………………………………... 34 Coral Reef Monitoring…………………………………………………………….. 13 References………………………………………………………………………………... 35 Monitoring results: Fish communities ………………………......……………… 14 Appendix: Species Lists……………………………………………………………….. 36 Monitoring results: Sea urchins …………………………………………………. 15 Monitoring results: Crown-of-thorns starfish …………………………………… 16 Seagrass monitoring……………………………………………………………… 17 Closed Forest Habitat (CFH) ……………………………………………………. 18 Ader’s Duiker………………………………………………………………………..19 Coconut -

Pacific Reef Assessment and Monitoring Program Data Report
Pacific Reef Assessment and Monitoring Program Data Report Ecological monitoring 2012–2013—reef fishes and benthic habitats of the main Hawaiian Islands, American Samoa, and Pacific Remote Island Areas A. Heenan1, P. Ayotte1, A. Gray1, K. Lino1, K. McCoy1, J. Zamzow1, and I. Williams2 1 Joint Institute for Marine and Atmospheric Research University of Hawaii at Manoa 1000 Pope Road Honolulu, HI 96822 2 Pacific Islands Fisheries Science Center National Marine Fisheries Service NOAA Inouye Regional Center 1845 Wasp Boulevard, Building 176 Honolulu, HI 96818 ______________________________________________________________ NOAA Pacific Islands Fisheries Science Center PIFSC Data Report DR-14-003 Issued 1 April 2014 This report outlines some of the coral reef monitoring surveys conducted by the National Oceanic and Atmospheric Administration (NOAA) Pacific Islands Fisheries Science Center’s Coral Reef Ecosystem Division in 2012 and 2013. This includes the following regions: American Samoa, the main Hawaiian Islands and the Pacific Remote Island Areas. 2 Acknowledgements Thanks to all those onboard the NOAA ships Hi`ialakai and Oscar Elton Sette for their logistical and field support during the 2012-2013 Pacific Reef Assessment and Monitoring Program (Pacific RAMP) research cruises and to the following divers for their assistance with data collection; Senifa Annandale, Jake Asher, Marie Ferguson, Jonatha Giddens, Louise Giuseffi, Mark Manuel, Marc Nadon, Hailey Ramey, Ben Richards, Brett Schumacher, Kosta Stamoulis and Darla White. We thank Rusty Brainard for his tireless support of Pacific RAMP and the staff of NOAA PIFSC CRED for assistance in the field and data management. This work was funded by the NOAA Coral Reef Conservation Program and the Pacific Islands Fisheries Science Center. -

Chumbe Island Management Plan 2017-2027
CHUMBE ISLAND rd 3 Ten Year Management Plan 2017 - 2027 This document is the third ten-year management plan for Chumbe Island Coral Park in Zanzibar, Tanzania. The two previous management plans covered the periods of 1995 to 2005, and 2006 to 2016 respectively. 2027 Goal The Chumbe Island Coral Reef Sanctuary and Closed Forest Reserve are effectively and sustainably managed in order to maximize their contribution to biodiversity conservation, serve as a model for effective ecotourism and MPA management, and provide a platform to promote wider environmental awareness for sustainable development and ecological stewardship in Zanzibar. Produced with support from: Sustainable Solutions International Consulting (SSIC) 2 Published by: Chumbe Island Coral Park (CHICOP) Citation: CHICOP (2017) 3rd Ten Year Management Plan for Chumbe Island Coral Park. Photos & images: Citations provided throughout document where required. All images permissible for use through creative commons or associated licensing, and/or direct owner consent. Cover photo: © CHICOP Design & layout: Sustainable Solutions International Consulting Available from: CHICOP, Zanzibar, Tanzania. E: [email protected] T: +255 (0) 242 231 040 3rd Ten Year Management Plan 2017 – 2027 Chumbe Island Coral Park 3 Contents Acronyms and Abbreviations .................................................................................................................. 6 Figures .................................................................................................................................................... -

American Samoa Archipelago Fishery Ecosystem Plan 2019
ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT: AMERICAN SAMOA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2019 Western Pacific Regional Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 PHONE: (808) 522-8220 FAX: (808) 522-8226 www.wpcouncil.org The ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT for the AMERICAN SAMOA FISHERY ECOSYSTEM PLAN 2019 was drafted by the Fishery Ecosystem Plan Team. This is a collaborative effort primarily between the Western Pacific Regional Fishery Management Council (WPRFMC), National Marine Fisheries Service (NMFS)-Pacific Island Fisheries Science Center (PIFSC), Pacific Islands Regional Office (PIRO), Hawaii Division of Aquatic Resources (HDAR), American Samoa Department of Marine and Wildlife Resources (DMWR), Guam Division of Aquatic and Wildlife Resources (DAWR), and Commonwealth of the Mariana Islands (CNMI) Division of Fish and Wildlife (DFW). This report attempts to summarize annual fishery performance looking at trends in catch, effort and catch rates as well as provide a source document describing various projects and activities being undertaken on a local and federal level. The report also describes several ecosystem considerations including fish biomass estimates, biological indicators, protected species, habitat, climate change, and human dimensions. Information like marine spatial planning and best scientific information available for each fishery are described. This report provides a summary of annual catches relative to the Annual Catch Limits established by the Council in collaboration with the local fishery management agencies. Edited By: Thomas Remington, Contractor & Marlowe Sabater and Asuka Ishizaki, WPRFMC. Cover image: credit to Clay Tam This document can be cited as follows: WPRFMC, 2020. Annual Stock Assessment and Fishery Evaluation Report for the American Samoa Archipelago Fishery Ecosystem Plan 2019. -

NBSREA Design Cvrs V2.Pub
February 2009 TNC Pacific Island Countries Report No 1/09 Rapid Ecological Assessment Northern Bismarck Sea Papua New Guinea Technical report of survey conducted August 13 to September 7, 2006 Edited by: Richard Hamilton, Alison Green and Jeanine Almany Supported by: AP Anonymous February 2009 TNC Pacific Island Countries Report No 1/09 Rapid Ecological Assessment Northern Bismarck Sea Papua New Guinea Technical report of survey conducted August 13 to September 7, 2006 Edited by: Richard Hamilton, Alison Green and Jeanine Almany Published by: The Nature Conservancy, Indo-Pacific Resource Centre Author Contact Details: Dr. Richard Hamilton, 51 Edmondstone Street, South Brisbane, QLD 4101 Australia Email: [email protected] Suggested Citation: Hamilton, R., A. Green and J. Almany (eds.) 2009. Rapid Ecological Assessment: Northern Bismarck Sea, Papua New Guinea. Technical report of survey conducted August 13 to September 7, 2006. TNC Pacific Island Countries Report No. 1/09. © 2009, The Nature Conservancy All Rights Reserved. Reproduction for any purpose is prohibited without prior permission. Cover Photo: Manus © Gerald Allen ISBN 9980-9964-9-8 Available from: Indo-Pacific Resource Centre The Nature Conservancy 51 Edmondstone Street South Brisbane, QLD 4101 Australia Or via the worldwide web at: conserveonline.org/workspaces/pacific.island.countries.publications ii Foreword Manus and New Ireland provinces lie north of the Papua New Guinea mainland in the Bismarck Archipelago. More than half of the local communities in our provinces are coastal inhabitants, who for thousands of years have depended on marine resources for their livelihood. For coastal communities survival and prosperity is integrally linked to healthy marine ecosystems. -
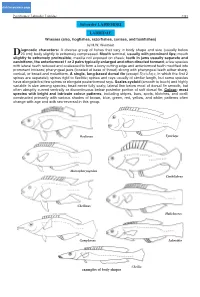
Suborder LABROIDEI LABRIDAE
click for previous page Perciformes: Labroidei: Labridae 3381 Suborder LABROIDEI LABRIDAE Wrasses (also, hogfishes, razorfishes, corises, and tuskfishes) by M.W. Westneat iagnostic characters: A diverse group of fishes that vary in body shape and size (usually below D20 cm); body slightly to extremely compressed. Mouth terminal, usually with prominent lips; mouth slightly to extremely protrusible; maxilla not exposed on cheek; teeth in jaws usually separate and caniniform, the anteriormost 1 or 2 pairs typically enlarged and often directed forward; a few species with lateral teeth reduced and coalesced to form a bony cutting edge and anteriormost teeth modified into prominent incisors; pharyngeal jaws (located at base of throat) strong with pharyngeal teeth either sharp, conical, or broad and molariform. A single, long-based dorsal fin (except Xyrichtys, in which the first 2 spines are separate); spines rigid to flexible; spines and rays usually of similar length, but some species have elongate first few spines or elongate posteriormost rays. Scales cycloid (smooth to touch) and highly variable in size among species; head never fully scaly; lateral line below most of dorsal fin smooth, but often abruptly curved ventrally or discontinuous below posterior portion of soft dorsal fin. Colour: most species with bright and intricate colour patterns, including stripes, bars, spots, blotches, and ocelli constructed primarily with various shades of brown, blue, green, red, yellow, and white; patterns often change with age and with sex-reversal in this group. Bodianus Xyrichtys Macropharyngodon Cirrhilabrus Cheilinus Halichoeres Gomphosus Labroides Cheilio examples of body shapes 3382 Bony Fishes Habitat, biology, and fisheries: Labrids are most common in shallow waters in a variety of habitats such as coral reefs, rocky reefs, sand, grass, and algae. -

Checklist of the Species of the Families Labridae and Scaridae: an Update
PARENTI & RANDALL Checklist of Labridae and Scaridae 29 Checklist of the species of the families Labridae and Scaridae: an update Paolo Parenti1 and John E. Randall2 1University of Milano–Bicocca, Department of Environmental Sciences, Piazza della Scienza, 1, 20126 Milano, Italy. Email: [email protected] 2Bishop Museum, 1525 Bernice St., Honolulu, HI 96817–2704, USA Email: [email protected] Recieved 7 July, accepted 1 October 2010 ABSTRACT. The checklist of the species of the RÉSUMÉ. Le répertoire des espèces des familles families Labridae and Scaridae is updated. des Labridae et des Scaridae est mis à jour. Après After the publication of the annotated checklist la publication du répertoire annoté (Parenti & (Parenti & Randall 2000) the species of Labridae Randall, 2000), les espèces Labridae ont augmenté increased from 453 to 504 and genera from 68 to 70, de 453 à 504 et les genres de 68 à 70, tandis que les whereas Scaridae increased from 88 to 99 species. Scaridae sont passées de 88 à 99 espèces. Altogether this account lists 53 species that are new Dans l’ensemble, ce comptage dénombre 53 espèces to science, while 14 species have been resurrected qui sont nouvelles à la science, tandis que 14 ont été from synonymy and 5 are now regarded as junior ressuscitées des synonymes et 5 sont actuellement synonyms. Comments on the status of 20 nominal considérées sous 5 nouveaux synonymes. Le species and notes on undescribed species are also répertoire comprend également des commentaires included. sur l’état de 20 espèces et des notes d’explication des espèces non encore décrites. -

A Pictorial Review of the Indo-Pacific Labrid Fish Genus Pseudocoris, with Description of a New Species from the Coral Sea
aqua, International Journal of Ichthyology A pictorial review of the Indo-Pacific labrid fish genus Pseudocoris, with description of a new species from the Coral Sea John E. Randall 1 and Fenton Walsh 2 1) Bishop Museum, 1525 Bernice St., Honolulu, HI 96817-2704, USA Email: [email protected] 2) Northern Barrier Marine Life, 29 Miles St., Cairns, Qld. 4870, Australia Email: [email protected] Received: 26 October 2007 – Accepted: 18 January 2008 Abstract phases adultes. Des photos couleurs sont fournies, de Pseudocoris aequalis is described as a new species of labrid juvéniles, de mâles en phase initiale et en phase terminale, fish collected from 8-15 m depth at Holmes Reef in the des cinq autres espèces du genre, avec des notes sur leur Coral Sea. The description is based on six specimens, four as distribution. Pseudocoris aurantiofasciata est signalé, pour terminal males, 100-112 mm SL, and two as males in the la première fois, en Australie, sur base d’un mâle en phase initial phase, 82 and 89 mm SL. The new species is distinct terminale, collecté à Holmes Reef. in lacking an elevated anterior part of the dorsal in the ter - minal-male phase, and the color pattern of both adult Sommario phases. Color photographs are provided of the juveniles, ini - Pseudocoris aequalis è descritta come nuova specie di tial-phase, and terminal males of the five other species of the labride raccolta a profondità di 8-15 m a Holmes Reef nel genus, with notes on their distribution. Pseudocoris aurantio - Mar dei Coralli.