NBSREA Design Cvrs V2.Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Guide to the Nonindigenous Marine Fishes of Florida
Field Guide to the Nonindigenous Marine Fishes of Florida Schofield, P. J., J. A. Morris, Jr. and L. Akins Mention of trade names or commercial products does not constitute endorsement or recommendation for their use by the United States goverment. Pamela J. Schofield, Ph.D. U.S. Geological Survey Florida Integrated Science Center 7920 NW 71st Street Gainesville, FL 32653 [email protected] James A. Morris, Jr., Ph.D. National Oceanic and Atmospheric Administration National Ocean Service National Centers for Coastal Ocean Science Center for Coastal Fisheries and Habitat Research 101 Pivers Island Road Beaufort, NC 28516 [email protected] Lad Akins Reef Environmental Education Foundation (REEF) 98300 Overseas Highway Key Largo, FL 33037 [email protected] Suggested Citation: Schofield, P. J., J. A. Morris, Jr. and L. Akins. 2009. Field Guide to Nonindigenous Marine Fishes of Florida. NOAA Technical Memorandum NOS NCCOS 92. Field Guide to Nonindigenous Marine Fishes of Florida Pamela J. Schofield, Ph.D. James A. Morris, Jr., Ph.D. Lad Akins NOAA, National Ocean Service National Centers for Coastal Ocean Science NOAA Technical Memorandum NOS NCCOS 92. September 2009 United States Department of National Oceanic and National Ocean Service Commerce Atmospheric Administration Gary F. Locke Jane Lubchenco John H. Dunnigan Secretary Administrator Assistant Administrator Table of Contents Introduction ................................................................................................ i Methods .....................................................................................................ii -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Capture, Identification and Culture Techniques of Coral Reef Fish Larvae
COMPONENT 2A - Project 2A1 PCC development February 2009 TRAINING COURSE REPORT CCapture,apture, iidentidentifi ccationation aandnd ccultureulture ttechniquesechniques ooff ccoraloral rreefeef fi sshh llarvaearvae ((FrenchFrench PPolynesia)olynesia) AAuthor:uthor: VViliameiliame PitaPita WaqalevuWaqalevu Photo credit: Eric CLUA The CRISP Coordinating Unit (CCU) was integrated into the Secretariat of the Pacifi c Community in April 2008 to insure maximum coordination and synergy in work relating to coral reef management in the region. The CRISP programme is implemented as part of the policy developed by the Secretariat of the Pacifi c Regional Environment Programme for a contribution to conservation and sustainable development of coral reefs in the Pacifi c he Initiative for the Protection and Management The CRISP Programme comprises three major compo- T of Coral Reefs in the Pacifi c (CRISP), sponsored nents, which are: by France and prepared by the French Development Agency (AFD) as part of an inter-ministerial project Component 1A: Integrated Coastal Management and from 2002 onwards, aims to develop a vision for the Watershed Management future of these unique ecosystems and the communi- - 1A1: Marine biodiversity conservation planning ties that depend on them and to introduce strategies - 1A2: Marine Protected Areas and projects to conserve their biodiversity, while de- - 1A3: Institutional strengthening and networking veloping the economic and environmental services - 1A4: Integrated coastal reef zone and watershed that they provide both locally and globally. Also, it is management designed as a factor for integration between deve- Component 2: Development of Coral Ecosystems loped countries (Australia, New Zealand, Japan and - 2A: Knowledge, benefi cial use and management USA), French overseas territories and Pacifi c Island de- of coral ecosytems veloping countries. -

Estimates of Sponge Consumption Rates on an Indo-Pacific Reef
Vol. 672: 123–140, 2021 MARINE ECOLOGY PROGRESS SERIES Published August 19 https://doi.org/10.3354/meps13786 Mar Ecol Prog Ser Estimates of sponge consumption rates on an Indo-Pacific reef Charlotte Mortimer1, Matthew Dunn2, Abdul Haris3, Jamaluddin Jompa3, James Bell1,* 1School of Biological Sciences, Victoria University of Wellington, Wellington 6012, New Zealand 2The National Institute of Water and Atmospheric Research (NIWA), Wellington 6021, New Zealand 3Universitas Hasanuddin, Department of Marine Science, Makassar 90245, Indonesia ABSTRACT: Determining predator diets is essential for understanding the strength of top-down processes and how they cascade through food webs. This is especially important for sponges, key members of benthic communities, whose dominance has increased in recent years on some coral reefs. However, the diversity of spongivorous fishes and the sponges they consume are relatively unknown. Here, we estimated sponge consumption by spongivorous fishes in the Wakatobi Marine National Park, Indonesia. We deployed cameras to identify fish biting at the dominant reef sponge Xestospongia spp. and then used gut content analysis and fish abundance estimates to quantify sponge consumption. In total, 33 species from 10 families of reef fish were identified taking bites from Xestospongia spp.; however, the 2 most prolific sponge-grazers, Ctenochaetus binotatus and Chaetodon kleinii, had no sponge in their guts, showing that for some fish, bites on sponge surfaces are not reliable evidence of sponge consumption. Gut contents indicated that Pygoplites diacanthus was an obligate spongivore, while Pomacanthus imperator, P. xanthometo- pon, Zanclus cornutus and Siganus punctatus regularly consumed sponges. Sponge consumption by these 5 spongivores was estimated at 46.6 ± 18.3 g sponge 1000 m−2 d−1. -

Reproductive Biology of Acanthurus Coeruleus (Bloch & Schneider, 1801) (Perciformes: Acanthuridae) in the North Coast of the State of Pernambuco, Brazil
Reproductive biology of Acanthurus coeruleus (Bloch & Schneider, 1801) (Perciformes: Acanthuridae) in the north coast of the State of Pernambuco, Brazil RAILMA MARIA VILANOVA QUEIROZ1, MARIANA GOMEZ DO RÊGO2, FABIO HISSA VIEIRA HAZIN1 & PAULO GUILHERME VASCONCELOS DE OLIVEIRA1 1 Universidade Federal Rural de Pernambuco, Departamento de Pesca e Aqüicultura, Av. Dom Manoel de Medeiros s/n, Dois Irmãos, 52171-900, Recife, Pernambuco, Brasil. 2 Universidade Federal Rural de Pernambuco, Departamento de Morfologia e Fisiologia Animal, Av. Dom Manoel de Medeiros s/n, Dois Irmãos, 52171-900, Recife, Pernambuco, Brasil. *Corresponding author: [email protected] Abstract. The objective of this work was to determine aspects of reproductive biology of Blue tang surgeonfish, Acanthurus coeruleus. 496 specimens were sampled, between January 2013 and December 2015, out of which, 235 were male and 261 were female. Sexual ratio did not significanlty differ among genders, and a ratio of 1.11 females per 1 male was found. Total length frequency distribution ranged from 14 to 41.7 cm with predominance of females in the 24-32 cm classes and of males in the 26- 32 cm classes. The estimated allometric coefficient (2.7695), suggests that the species presents negative allometric growth. According to the GSI and the gonads histological analysis, the reproductive period of the species occurs from June to January with periods of greater reproductive activity from August. The oocyte diameter-frequency distribution analysis revealed a multimodal distribution, confirming the evidence of multiple spawning and batch fecundity varied from 20000 to 55000 oocytes. Spawning frequency estimates, based on the hydrated oocytes (HO) method indicated that the species spawns once every 3.8 days, while the estimates based on the post-ovulatory follicle (POF) method indicated a spawning every 3.4 days, during a 6-month spawning season lasting from August to January. -

South-West Pacific Node Training 12-16 November 2007
COMPONENT 2A - Project 2A2 Knowledge, monitoring, management and benefi cial use of coral reef ecosystems April 2008 REEF MONITORING SOUTH-WEST PACIFIC NODE TRAINING 12-16 NOVEMBER 2007 Author: Naushad YAKUB The CRISP programme is implemented as part of the policy developped by the Secretariat of the Pacifi c Regional Environment Programme for a contribution to conservation and sustainable development of coral reefs in the Pacifi c he Initiative for the Protection and Management of Coral Reefs in the Pacifi c T (CRISP), sponsored by France and prepared by the French Development Agency (AFD) as part of an inter-ministerial project from 2002 onwards, aims to develop a vision for the future of these unique eco-systems and the communities that depend on them and to introduce strategies and projects to conserve their biodiversity, while developing the economic and environmental services that they provide both locally and globally. Also, it is designed as a factor for integration between developed countries (Australia, New Zealand, Japan and USA), French overseas territories and Pacifi c Island developing countries. The CRISP Programme comprises three major components, which are: Component 1A: Integrated Coastal Management and Watershed Management - 1A1: Marine biodiversity conservation planning - 1A2: Marine Protected Areas - 1A3: Institutional strengthening and networking - 1A4: Integrated coastal reef zone and watershed management CRISP Coordinating Unit (CCU) Component 2: Development of Coral Ecosystems Programme manager: Eric CLUA - 2A: -

Monitoring Functional Groups of Herbivorous Reef Fishes As Indicators of Coral Reef Resilience a Practical Guide for Coral Reef Managers in the Asia Pacifi C Region
Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience A practical guide for coral reef managers in the Asia Pacifi c Region Alison L. Green and David R. Bellwood IUCN RESILIENCE SCIENCE GROUP WORKING PAPER SERIES - NO 7 IUCN Global Marine Programme Founded in 1958, IUCN (the International Union for the Conservation of Nature) brings together states, government agencies and a diverse range of non-governmental organizations in a unique world partnership: over 100 members in all, spread across some 140 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. The IUCN Global Marine Programme provides vital linkages for the Union and its members to all the IUCN activities that deal with marine issues, including projects and initiatives of the Regional offices and the six IUCN Commissions. The IUCN Global Marine Programme works on issues such as integrated coastal and marine management, fisheries, marine protected areas, large marine ecosystems, coral reefs, marine invasives and protection of high and deep seas. The Nature Conservancy The mission of The Nature Conservancy is to preserve the plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Conservancy launched the Global Marine Initiative in 2002 to protect and restore the most resilient examples of ocean and coastal ecosystems in ways that benefit marine life, local communities and economies. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -
![Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]](https://docslib.b-cdn.net/cover/7255/ornamental-fish-and-marine-invertebrates-draft-for-consultation-document-date-1037255.webp)
Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]
Ornamental Fish and Marine Invertebrates ORNAMARI.ALL [Document Date] Health Standard Import Import Issued under the Biosecurity Act 1993 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] TITLE Import Health Standard: Ornamental Fish and Marine Invertebrates COMMENCEMENT This Import Health Standard comes into force on [Effective Date] REVOCATION This Import Health Standard revokes and replaces: Import Health Standard for Ornamental Fish and Marine Invertebrates from all countries, 20 April 2011. ISSUING AUTHORITY This Import Health Standard is issued on Dated at Wellington this ... day of ......... Howard Pharo Manager, Import and Export Animals Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Regulation & Assurance Branch Animal Imports PO Box 2526 Wellington 6140 Email: [email protected] Ministry for Primary Industries Page 1 of 75 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] Contents Page Introduction 4 Part 1: Requirements 6 1.1 Application 6 1.2 Outcome 6 1.3 Incorporation by reference 7 1.4 Definitions 7 1.5 Harmonised system (HS) codes 7 1.6 Exporting country systems and certification 8 1.7 Diagnostic testing and treatment 8 1.8 Packaging 9 1.9 Import permit 9 1.10 The documentation that must accompany goods 9 1.11 Inspection and verification 10 1.12 Transitional facility 11 1.13 Pre-export isolation -

Ecological Relationships in Hawaiian and Johnston Island Acanthuridae (Surgeonfishes)1
Ecological Relationships in Hawaiian and Johnston Island Acanthuridae (Surgeonfishes)1 Robert S. JONES Division of Biosciences and Marine Studies, University of Guam ABSTRACT Four genera and twenty species of the family Acanthuridae from Hawaii and Johnston Island are investigated for factors that might provide potential ecological separation of the species. The factors investigated a re habitat preference, foraging methods, food eaten, and possible morphological specializations for feeding. On the basis of habitat preference the acanthurids may be divided into mid-water, sand patch, subsurge reef, and seaward reef or surge zone dwellers. These habitats are defined in terms of acanthurid species .composition and general physiography. With regard to foraging methods and food eaten, the Acanthuridae comprise zooplankton feeders, grazers, and browsers. The zooplankton feeders actively pursue and capture copepods, crustacean larvae, and the pelagic eggs of numerous marine animals. Grazers feed predominate ly on a calcareous substratum rich in diatoms and detritus. The browsers feed on multicellular benthic algae of two basic types based on the size and morphology of the a lgae. At the species level several modifications in the morphology of the digestive tract are found which suggest that many of these fishes are able to handle their food in a different manner from other species. INTRODUCTION One of the largest groups of reef fishes in terms of species and biomass in the Hawaiian and Johnston Island marine environments is the family Acanthuri dae. For the most part the species of this family are wide-spread and frequently the most abundant of the diurnally active fishes in the littoral waters of these localities. -

Journal of Ichthyology and Aquatic Biology Vol
aqua Journal of Ichthyology and Aquatic Biology Vol. 4 (3), April 2001 Aquapress ISSN 0945-9871 aqua - Journal of Ichthyology and Aquatic Biology Managing Editor: Scope and aims Heiko Bleher Via G. Falcone 11 - 27010 Miradolo Terme (PV) - Italy aqua is an international journal which publishes original Tel.: +39 0382 754707/08 - Fax: +39 0382 754129 scientific articles in the fields of systematics, taxonomy, e-mail: [email protected] biogeography, ethology, ecology, and general biology of fishes, amphibians, aquatic invertebrates, and plants. Scientific Editor: Papers on freshwater, brackish, and marine organisms Dr. Walter Ivantsoff will be considered. aqua is fully refereed and aims at Senior Research Fellow publishing manuscripts within 2-4 months of acceptance. Department of Biological Sciences With the publication of aqua we are pursuing a new con - Macquarie University N.S.W. 2109 - Australia cept: this scientific journal is being issued parallel to e-mail: [email protected] aqua geõgraphia , an international magazine which pre - Tel. +61 2 9850 8167 - Fax +61 2 9850 8245 sents life above and in the water. The simultaneous pub - lication of a popular and a scientific periodical will guar - antee a high number of copies and a wide distribution at Editorial Board: a low price. In view of the importance of colour patterns Gerald R. Allen - I Dreyer Road Roleystone, in species identification and animal ethology, authors are W.A. Australia 6111 encouraged to submit colour illustrations as well as descriptions of coloration. It is our aim to provide Henri J. Dumont, Rijksuniversiteit Gent, Laboratorium the international scientific community with an efficiently voor Ecologie der Dieren, Zoogeografie en Natuur- published series meeting high scientific and technical behoud, K. -
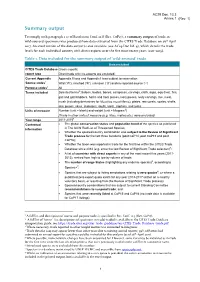
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g.