Diagnostic and Interventional Venous Procedures (Lower Extremity)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deep Venous Thrombosis with Suspected Pulmonary Embolism
Thoracic Imaging Peter A. Loud, MD Deep Venous Thrombosis Douglas S. Katz, MD Dennis A. Bruce, MD with Suspected Pulmonary Donald L. Klippenstein, MD Zachary D. Grossman, MD Embolism: Detection with Combined CT Venography Index terms: Computed tomography (CT), 1 angiography, 9*.129142, and Pulmonary Angiography 9*.12915, 9*.12916 Embolism, pulmonary, 60.72 Pulmonary angiography, 944.12914, 944.12915, 944.12916 PURPOSE: To determine the frequency and location of deep venous thrombosis at Veins, thrombosis, 9*.751, 9*.12914 computed tomographic (CT) venography after CT pulmonary angiography in a large series of patients clinically suspected of having pulmonary embolism and to Radiology 2001; 219:498–502 compare the accuracy of CT venography with lower-extremity venous sonography. Abbreviation: MATERIALS AND METHODS: Venous phase images were acquired from the DVT ϭ deep venous thrombosis diaphragm to the upper calves after completion of CT pulmonary angiography in 650 patients (373 women, 277 men; age range, 18–99 years; mean age, 63 years) 1 From the Department of Radiology, to determine the presence and location of deep venous thrombosis. Results of CT Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY 14263 (P.A.L., venography were compared with those of bilateral lower-extremity venous sonog- D.L.K., Z.D.G.), and the Department raphy in 308 patients. of Radiology, Winthrop University Hospital, Mineola, NY (D.S.K., D.A.B.). RESULTS: A total of 116 patients had pulmonary embolism and/or deep venous From the 1999 RSNA scientific assem- thrombosis, including 27 patients with pulmonary embolism alone, 31 patients with bly. -

Spring Programme 2011
Faculty of Radiologists Royal College of Surgeons in Ireland Combined Spring Meeting 8th & 9 th April 2011 Venue: Castlemartyr Hotel, Co. Cork. Programme Faculty of Radiologists, Royal College of Surgeons in Ireland CPD Credits Awarded: 5 Royal College of Radiologists credits are applied for. Friday 8 th April 2011 3.30-4.30pm Registration 4.30-5.30pm Stroke in 2011, Moderator: Dr. Ian Kelly, Waterford Regional Hospital 4.30-5.00pm Acute Stoke Imaging. Dr Noel Fanning, Cork University Hospital, Cork 5.00-5.30pm Stroke: A clinical perspective. Dr. George Pope, John Radcliffe Hospitals, Oxford 5.30-6.30pm Moderator: Dr. Adrian Brady, Dean, Faculty of Radiologists Belfast to Bosnia and Autopsy to Virtopsy Dr. Jack Crane, State Pathologist, NI 8pm Dinner Saturday 9 th April 2011 8.30-9.00am Registration 9.00-10.00am Liver hour. Moderator: Dr John Feeney, AMNCH, Dublin 9.00-9.30am Liver imaging pre metastatectomy. Dr. Peter MacEneaney, Mercy University Hospital, Cork 9.30-10.00am Parenchymal and focal liver biopsy - when and how. Dr Stephen J Skehan St Vincent's University Hospital, Dublin 10.00-11.00am Paediatric Hour. Moderator: Dr. Stephanie Ryan, The Children’s University Hospital Temple Street, Dublin 10.00-10.30am Paediatric Abdominal Emergencies. Dr Eoghan Laffan, The Children’s, University Hospital Temple Street, Dublin 10.30-11.00am Non Accidental Injury. Dr Conor Bogue, Cork University Hospital, Cork 11.00-11.30am Tea/Coffee Break and Poster Exhibition 11.30-12.30pm MSK Hour. Moderator: Dr Orla Buckley, AMNCH, Dublin 11.30-12.00pm Image guided joint interventions. -
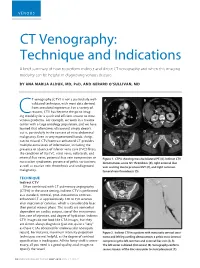
CT Venography: Technique and Indications
VENOUS CT Venography: Technique and Indications A brief summary of how to perform indirect and direct CT venography and when this imaging modality can be helpful in diagnosing venous disease. BY ANA MARIJA ALDUK, MD, PHD, AND GERARD O’SULLIVAN, MD T venography (CTV) is not a particularly well- A B validated technique, with most data derived from anecdotal experience. For a variety of reasons, CTV has become the go-to imag- Cing modality for a quick and efficient answer to most venous problems. For example, we work in a trauma center with a large oncology population, and we have learned that oftentimes ultrasound simply doesn’t cut it, particularly in the context of intra-abdominal C D malignancy. Even in very experienced hands, things can be missed. CTV/contrast-enhanced CT provides multiple extra levels of information, including the presence or absence of inferior vena cava (IVC) filters; the condition of the IVC, renal veins, collaterals, and internal iliac veins; potential iliac vein compression or Figure 1. CTPA showing massive bilateral PE (A). Indirect CTV nutcracker syndromes; presence of pelvic varicosities; demonstrates acute IVC thrombosis (B), right external iliac as well as ovarian vein thrombosis and undiagnosed vein scarring due to previous DVT (C), and right common malignancy. femoral vein thrombosis (D). TECHNIQUE A C Indirect CTV Often combined with CT pulmonary angiography (CTPA) in the acute setting, indirect CTV is performed as a standard, nonoral, post–intravenous contrast- enhanced CT at approximately 120 to 150 seconds after injection of contrast, which is considerably later than portal venous phase. -

2Nd Quarter 2001 Medicare Part a Bulletin
In This Issue... From the Intermediary Medical Director Medical Review Progressive Corrective Action ......................................................................... 3 General Information Medical Review Process Revision to Medical Record Requests ................................................ 5 General Coverage New CLIA Waived Tests ............................................................................................................. 8 Outpatient Hospital Services Correction to the Outpatient Services Fee Schedule ................................................................. 9 Skilled Nursing Facility Services Fee Schedule and Consolidated Billing for Skilled Nursing Facility (SNF) Services ............. 12 Fraud and Abuse Justice Recovers Record $1.5 Billion in Fraud Payments - Highest Ever for One Year Period ........................................................................................... 20 Bulletin Medical Policies Use of the American Medical Association’s (AMA’s) Current Procedural Terminology (CPT) Codes on Contractors’ Web Sites ................................................................................. 21 Outpatient Prospective Payment System January 2001 Update: Coding Information for Hospital Outpatient Prospective Payment System (OPPS) ......................................................................................................................... 93 he Medicare A Bulletin Providers Will Be Asked to Register Tshould be shared with all to Receive Medicare Bulletins and health care -

(MRA) and Magnetic Resonance Venography (MRV) Medical Policy
Magnetic Resonance Angiography (MRA) and Magnetic Resonance Venography (MRV) Medical Policy The content of this document is used by plans that do not utilize NIA review. Service: Magnetic Resonance Angiography (MRA) and Magnetic Resonance Venography (MRV) PUM 250-0027-1712 Medical Policy Committee Approval 12/11/2020 Effective Date 01/01/2021 Prior Authorization Needed Yes Description: Magnetic Resonance Angiography (MRA) and Magnetic Resonance Venography (MRV) use Magnetic resonance imaging (MRI) technology to produce detailed 2-dimensional or 3- dimensional images of the vascular system and may be tailored to assess arteries or veins. It is often used for vascular conditions where other types of imaging are considered inferior or contraindicated, and to decrease risk of cumulative radiation exposure and often instead of invasive procedures. Indications of Coverage: A. MRA/MRV is considered medically necessary for the anatomical regions listed below when the specific indications or symptoms described are documented: 1. Head/Brain: a. Suspected intracranial aneurysm (ICA) or arteriovenous malformation (AVM). Any of the following: 1. Acute severe headache, severe exertional headache, or sudden onset of explosive headache, in individuals with signs / symptoms highly suggestive of a leaking/ruptured internal carotid artery or arteriovenous malformation. 2. Known subarachnoid hemorrhage or diagnosis of spontaneous intracerebral hemorrhage with concern for underlying vascular abnormality. 3. Suspected arteriovenous malformation (AVM) or dural AV fistula in an individual with prior indeterminate imaging study 4. Thunderclap headache with question of underlying vascular abnormality AND prior negative workup to include EITHER i. negative brain MRI, OR ii. Negative brain CT and negative lumbar puncture Page 1 of 15 5. -
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Optos 200Tx and Heidelberg Spectralis
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 19, 2021 Performance evaluation of two fundus oculi angiographic imaging system: Optos 200Tx and Heidelberg Spectralis SHUANG LI, JING‑JING WANG, HONG‑YANG LI, WEI WANG, MENG TIAN, XU‑QIANG LANG and KANG WANG Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, P.R. China Received December 15, 2018; Accepted October 29, 2019 DOI: 10.3892/etm.2020.9451 Abstract. The present study aimed to compare the imaging Introduction performance of two ultra‑wide‑field fluorescein angiog‑ raphy imaging systems, namely the OptosOptomap 200Tx Ultra‑wide‑field fluorescein angiography (UWFA) is a novel (Optos 200Tx) and the Heidelberg Spectralis (Spectralis). A total technology that has developed rapidly in recent years (1,2). of 18 patients (36 eyes) underwent angiography using the two As numerous pathological changes of fundus diseases occur systems at the Department of Ophthalmology, Beijing Friendship at the edge of the retina, the limitation of imaging leads to Hospital (Beijing, China) between January and June 2017. The insufficient diagnosis or underestimation of the severity of the images were obtained as a single shot centered on the macula. disease (3,4). Therefore, clear imaging of the edge of the retina The total area and area within each of four visualized quadrants is important for the diagnosis, monitoring and prognostication were calculated and compared. The averages of the total and of patients with ocular fundus diseases. The traditional fundus individual quadrant area captured by the Optos 200Tx were fluorescein angiography system may only provide a vision field all larger than those obtained with the Spectralis (P<0.05). -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Initial Observations Comparing MDCT and 3.0T MRI Findings with Autopsy Findings
Utility of Postmortem Autopsy via Whole- Body Imaging: Initial Observations Comparing MDCT and 3.0T MRI Findings with Autopsy Findings Jang Gyu Cha, MD1 Dong Hun Kim, MD1 Objective: We prospectively compared whole-body multidetector computed Dae Ho Kim, MD2 tomography (MDCT) and 3.0T magnetic resonance (MR) images with autopsy Sang Hyun Paik, MD1 findings. Jai Soung Park, MD1 Materials and Methods: Five cadavers were subjected to whole-body, 16- Seong Jin Park, MD1 channel MDCT and 3.0T MR imaging within two hours before an autopsy. A radi- Hae Kyung Lee, MD1 ologist classified the MDCT and 3.0T MRI findings into major and minor findings, Hyun Sook Hong, MD1 which were compared with autopsy findings. 3 Duek Lin Choi, MD Results: Most of the imaging findings, pertaining to head and neck, heart and 4 Kyung Moo Yang, MD vascular, chest, abdomen, spine, and musculoskeletal lesions, corresponded to 4 Nak Eun Chung, MD autopsy findings. The causes of death that were determined on the bases of 4 Bong Woo Lee, MD MDCT and 3.0T MRI findings were consistent with the autopsy findings in four of 4 Joong Seok Seo, MD five cases. CT was useful in diagnosing fatal hemorrhage and pneumothorax, as well as determining the shapes and characteristics of the fractures and the direc- Index terms: tion of external force. MRI was effective in evaluating and tracing the route of a Computed tomography (CT) metallic object, soft tissue lesions, chronicity of hemorrhage, and bone bruises. Magnetic resonance (MR) Whole-body imaging Conclusion: A postmortem MDCT combined with MRI is a potentially powerful Forensic autopsy tool, providing noninvasive and objective measurements for forensic investiga- DOI:10.3348/kjr.2010.11.4.395 tions. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography.