Current State-Of-The-Art in Intravascular Coronary Imaging
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
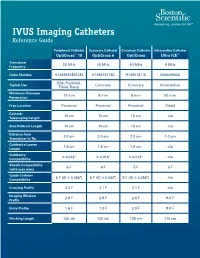
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography. -

Final Addenda FY 2005
FY 2005 Final Addenda ICD-9-CM Volume 3, Procedures Effective October 1, 2004 Tabular List 00.0 Therapeutic ultrasound Add exclusion term Excludes: diagnostic ultrasound (non-invasive) (88.71-88.79) intracardiac echocardiography [ICE] (heart chamber(s)) (37.28) intravascular imaging (adjunctive) (00.21-00.29) New code 00.16 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Ex-vivo treatment of vessel Hyperbaric pressurized graft [conduit] New code 00.17 Infusion of vasopressor agent New subcategory 00.2 Intravascular imaging of blood vessels Endovascular ultrasonography Intravascular ultrasound (IVUS) Code also any synchronous diagnostic or therapeutic procedures Excludes: therapeutic ultrasound (00.01-00.09) New code 00.21 Intravascular imaging of extracranial cerebral vessels Common carotid vessels and branches Intravascular ultrasound (IVUS), extracranial cerebral vessels Excludes: diagnostic ultrasound (non-invasive) of head and neck (88.71) New code 00.22 Intravascular imaging of intrathoracic vessels Aorta and aortic arch Intravascular ultrasound (IVUS), intrathoracic vessels Vena cava (superior) (inferior) Excludes: diagnostic ultrasound (non-invasive) of other sites of thorax (88.73) New code 00.23 Intravascular imaging of peripheral vessels Imaging of: vessels of arm(s) vessels of leg(s) Intravascular ultrasound (IVUS), peripheral vessels Excludes: diagnostic ultrasound (non-invasive) of peripheral vascular system (88.77) New code 00.24 Intravascular imaging of coronary vessels Intravascular -

Intravascular Ultrasound Appearance of Normal and Mildly Diseased Coronary Arteries: Correlation with Histologic Specimens
Intravascular ultrasound appearance of normal and mildly diseased coronary arteries: Correlation with histologic specimens Bavani Maheswaran, MD, Cyril Y. Leung, MD, Dan E. Gutfinger, PhD, Shigeru Nakamura, MD, Robert J. Russo, MD, Takafumi Hire, MD, and Jonathan M. Tobis, MD Irvine, Calif. Intravascular ultrasound imaging was performed in vitro on vestigate the histologic tissue characteristics that six histologically normal and 104 minimally diseased art- determine the ultrasound representation of coronary eries in patients aged 13 to 83 years. This study tested the artery microanatomy. hypothesis that normal coronary arteries produce a three- layer image that corresponds to the histologic layers of in- METHODS tima, media, and adventitia. The results showed a very good Human coronary arteries, Coronary artery segments correlation between area of the echolucent ultrasound layer with the media and the inner echogenic layer with intimal that appeared grossly normal by palpation were obtained area. In addition, a three-layer appearance was consistently at autopsy from 110 people over a wide age range. Because seen when the internal elastic membrane was present with the arteries were obtained from the coroner's office, little or without intimal hyperplasia. If the internal elastic mem- other clinical information was available. At the time of ul- brane was absent, a three-layer appearance was still seen if trasound imaging, the arteries were fresh and tmY-Lxed.An the collagen content of the media was low. However, a two- 8F guiding catheter was inserted into the proximal end of layer appearance was observed when there was absence of the vessel, and normal saline was perfused at 100 mm Hg the internal elastic membrane as well as a high collagen at room temperature in a saline bath. -

Imaging of Pulmonary Vascular Disease by Intravascular Ultrasound
International Journal of Cardiac Imaging 9: 179-184,1993. @ 1993 Kluwer Academic Publishers. Printed in the Netherlands. Imaging of pulmonary vascular disease by intravascular ultrasound Peter John Scott,1 Ahmed Rashid Essop, 1Waddah A1-Ashab, ~Andrew Deaner, 1Johnathan Parsons 2 & Gordon Williamsl ~Non-Invasive Heart Unit, 2Department of Paediatric Cardiology, Killingbeck Hospital, Leeds, West Yorkshire, UK (*corresponding address) Dept of Cardiology, Leeds General Infirmary, Great George Street, Leeds, West Yorkshire LSI 3EX, UK Accepted 4 January1993 Key words: pulmonary vascular disease, intravascular ultrasound Abstract To assess the ability of intravascular ultrasound (IVUS) to image changes in the pulmonary arterial wall associated with pulmonary hypertension (PHT), 10 subjects requiring diagnostic right and left heart cathe- terization were studied. In addition to measurements of pulmonary artery pressure and pulmonary vascular resistance and pulmonary angiography, when indicated, all underwent simultaneous IVUS imaging in the pulmonary arterial system using a 20MHz ultrasound transducer mounted on a 2 mm diameter catheter. Four patients had normal pulmonary artery pressures and 6 had varying degrees of PHT. Satisfactory ultrasound images were obtained in 9 out of the 10 patients. In those with normal pulmonary artery pressures ultrasound showed a thin vessel wall with no destinction between separate layers. In patients with systemic PHT, a three- layered vessel wall was apparent and areas compatible with intimal proliferation were seen. In a patient with pulmonary embolic disease areas consistent with mural thrombus were detected at sites of luminal narrowing on the pulmonary angiogram. IVUS is capable of imaging some of the morphological changes in the wall of the pulmonary artery known to occur in longstanding PHT and may therefore become a useful adjunct to haemodynamicmeasurements and pulmonary angiography for the in vivo assessement of pulmonaryvascular disease. -

Usefulness of Intravascular Ultrasonography Monitoring of Coil Embolization for Traumatic Direct Carotid-Cavernous Fistula —Case Report—
Neurol Med Chir (Tokyo) 49, 604¿607, 2009 Usefulness of Intravascular Ultrasonography Monitoring of Coil Embolization for Traumatic Direct Carotid-Cavernous Fistula —Case Report— Akimasa NISHIO, Taichiro KAWAKAMI,YutakaMITSUHASHI,KojiHAYASAKI*, Miki KIYAMA**,YuichiTADA**,andKenjiOHATA Department of Neurosurgery, Osaka City University Graduate School of Medicine, Osaka; *Department of Neurosurgery, Saiseikai Ibaraki Hospital, Ibaraki, Osaka; **Department of Neurosurgery, Ouekai Katsuragi Hospital, Kishiwada, Osaka Abstract A 61-year-old woman was admitted for head injury after a traffic accident. Two months later, she devel- oped abducens nerve palsy, chemosis, and pulsatile tinnitus. Right internal carotid angiography demonstrated a high flow direct carotid-cavernous fistula (CCF) at the C5 portion with reflux into the su- perficial and deep sylvian veins, superior ophthalmic vein, superior petrosal sinus, and inferior petrosal sinus. Intravascular ultrasonography (IVUS) revealed a large fistula at the C5 portion of the in- ternal carotid artery (ICA). Coil embolization via transarterial and transvenous approaches under IVUS monitoring was performed. During the procedure, IVUS accurately detected protrusion of a coil into the parent ICA, and the parent artery could be preserved. IVUS monitoring is useful for embolization of direct CCF with coils. Key words: direct carotid-cavernous fistula, embolization, intravascular ultrasound Introduction paresis. In addition, the coil mass may protrude into the parent artery through the large tear or may obscure the Direct carotid-cavernous fistulas (CCFs) are preferably relationship between cavernous sinus and the nearby par- treated via a transarterial approach, ideally resulting in ent artery, with increased risk of occluding the parent ar- permanent occlusion of the fistula and preservation of the tery. -

Diagnostic and Interventional Venous Procedures (Lower Extremity)
Coding and Medicare national payment guide 2018 Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips is gathered from third-party sources and is subject to change. The information is intended to serve as a general reference guide and does not constitute reimbursement or legal advice. For all coding, coverage and reimbursement matters or questions about the information contained in this material, Philips recommends that you consult with your payers, certified coders, reimbursement specialists and/or legal counsel. Philips does not guarantee that the use of any particular codes will result in coverage or payment at any specific level. Coverage for these procedures may vary by Payer. Philips recommends that providers verify coverage prior to date of service. This information may include some codes for procedures for which Philips currently offers no cleared or approved products. In those instances, such codes have been included solely in the interest of providing users with comprehensive coding information and are not intended to promote the use of any products. The selection of a code must reflect the procedure(s) documented in the medical record. Providers are responsible for determining medical necessity, the proper place of service, and for submitting accurate claims. Payment amounts set forth herein are 2018 Medicare national averages; local Medicare payment amounts and private payer rates will vary. Page 2 See page 2 for important information about the uses and limitations of this guide and page 10 for all third-party sources. Hospital inpatient 1 Hospitals are reimbursed by Medicare for inpatient procedures and services under the FY2018 Inpatient Prospective Payment System (IPPS), which utilizes the Medicare Severity Diagnosis Related Group (MS-DRG) system. -

Correlation of Intravascular Ultrasound with Histology in Pediatric Pulmonary Vein Stenosis
children Article Correlation of Intravascular Ultrasound with Histology in Pediatric Pulmonary Vein Stenosis Ryan Callahan 1,* , Zachary Gauthier 1 , Shuhei Toba 1,2, Stephen P. Sanders 1,2, Diego Porras 1 and Sara O. Vargas 2 1 Department of Cardiology, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115, USA; [email protected] (Z.G.); [email protected] (S.T.); [email protected] (S.P.S.); [email protected] (D.P.) 2 Department of Pathology, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115, USA; [email protected] * Correspondence: [email protected] Abstract: Preliminary intravascular ultrasound (IVUS) images of suspected pediatric intraluminal pulmonary vein stenosis (PVS) demonstrate wall thickening. It is unclear how the IVUS-delineated constituents of wall thickening correlate with the histology. We analyzed six postmortem formalin- fixed heart/lung specimens and four live patients with PVS as well as control pulmonary veins using IVUS and light microscopic examination. In PVS veins, IVUS demonstrated wall thickening with up to two layers of variable echogenicity, often with indistinct borders. Histologically, the veins showed fibroblastic proliferation with areas rich in myxoid matrix as well as areas with abundant collagen and elastic fibers. Discrete vein layers were obscured by scarring and elastic degeneration. A lower reflective periluminal layer by IVUS corresponded with hyperplasia of myofibroblast-like Citation: Callahan, R.; Gauthier, Z.; cells in abundant myxoid matrix. The hyper-reflective layer by IVUS extended to the outer edge Toba, S.; Sanders, S.P.; Porras, D.; Vargas, S.O. -

How Accurate Is Atherosclerosis Imaging by Coronary Computed Tomography Angiography?
UCLA UCLA Previously Published Works Title How accurate is atherosclerosis imaging by coronary computed tomography angiography? Permalink https://escholarship.org/uc/item/20d1m443 Journal Journal of cardiovascular computed tomography, 13(5) ISSN 1934-5925 Authors Nakanishi, Rine Motoyama, Sadako Leipsic, Jonathon et al. Publication Date 2019-09-01 DOI 10.1016/j.jcct.2019.06.005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Journal of Cardiovascular Computed Tomography xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Journal of Cardiovascular Computed Tomography journal homepage: www.elsevier.com/locate/jcct Invited review How accurate is atherosclerosis imaging by coronary computed tomography angiography? ∗ Rine Nakanishia, , Sadako Motoyamab, Jonathon Leipsicc, Matthew J. Budoffd a Department of Cardiovascular Medicine, Toho University Graduate School of Medicine, Tokyo, Japan b Department of Cardiology, Fujita Health University, Aichi, Japan c St. Paul's Hospital, University of British Columbia, Vancouver, British Columbia, Canada d Los Angeles BioMedical Research Institute at Harbor UCLA Medical Center, Torrance, CA, USA ARTICLE INFO ABSTRACT Keywords: Invasive coronary plaque imaging such as intravascular ultrasound and optical coherence tomography has been Coronary computed tomographic angiography widely used to observe culprit or non-culprit coronary atherosclerosis, as well as optimize stent sizing, appo- Intravascular ultrasound sition and deployment. Coronary computed -

ICD-10-PCS Reference Manual
ICD-10-PCS Reference Manual 3 Table of Contents Preface ............................................................................................ xi Manual organization ................................................................................................................. xi Chapter 1 - Overview ......................................................................................................... xi Chapter 2 - Procedures in the Medical and Surgical section ............................................. xi Chapter 3 - Procedures in the Medical and Surgical-related sections ............................... xi Chapter 4 - Procedures in the ancillary sections ............................................................... xii Appendix A - ICD-10-PCS definitions ................................................................................ xii Appendix B - ICD-10-PCS device and substance classification ........................................ xii Conventions used ..................................................................................................................... xii Root operation descriptions ............................................................................................... xii Table excerpts ................................................................................................................... xiii Chapter 1: ICD-10-PCS overview ......................................................... 15 What is ICD-10-PCS? .............................................................................................................