Intravascular Ultrasound Imaging in Evaluation Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -

Introduction
RIMS, IMPHAL ANNUAL REPORT 2014-15 INTRODUCTION 1. DESCRIPTION : The Regional Institute of Medical Sciences (RIMS), Imphal was established in the year 1972. It is an institution of regional importance catering to the needs of the North Eastern Region in the field of imparting undergraduate and post graduate medical education.The Institution brings together educational facilities for the training of personnel in all important branches of medical specialities including Dental and Nursing education in one place. The Institute is affiliated to the Manipur University, Canchipur, Imphal. 2. MANAGEMENT : The Institute was transferred to the Ministry of Health & Family Welfare, Government of India from North Eastern Council, Shillong (under Ministry of DoNER, Government of India) w.e.f. 1st April, 2007. Under the existing administrative set-up, the highest decision making body is the Board of Governors headed by the Union Minister of Health & Family Welfare as the President and the Director of the Institute as the Secretary. The Executive Council is responsible for the management of the Institute. The Secretary, Ministry of Health & Family Welfare, Government of India is the Chairman of the Executive Council while the head of the Institute remains as Secretary. Thus, the institute is managed at two levels, namely the Board of Governors and the Executive Council. A. Board of Governors : 1. Hon’ble Union Minister, - President Health & Family Welfare, Government of India. 2. Hon’ble Chief Minister, Manipur. - Vice-President 3. A Representative of the Planning Commission, - Member Government of India. 4. Health Ministers of the Beneficiary States - Member 5. Secretary, Ministry of Health & Family Welfare, - Member Government of India. -
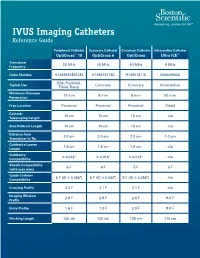
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Using Sound Advice—Intravascular Ultrasound As a Diagnostic Tool
Commentary Using sound advice—intravascular ultrasound as a diagnostic tool Yasir Parviz1, Khady N. Fall1, Ziad A. Ali1,2 1Center for Interventional Vascular Therapy, Division of Cardiology, Presbyterian Hospital and Columbia University, New York, USA; 2Cardiovascular Research Foundation, New York, USA Correspondence to: Ziad A. Ali. Center for Interventional Vascular Therapy, Division of Cardiology, Presbyterian Hospital and Columbia University, New York, NY, USA; Cardiovascular Research Foundation, New York, NY, USA. Email: [email protected]. Submitted Sep 06, 2016. Accepted for publication Sep 08, 2016. doi: 10.21037/jtd.2016.10.64 View this article at: http://dx.doi.org/10.21037/jtd.2016.10.64 Intravascular ultrasound (IVUS) uses varying-frequency (6.0% vs. 13.6%) (5). catheter-based transducers for assessment of blood vessel By extrapolation, IVUS may also have utility in the dimensions and morphology. Along with advances in the emergency setting for pathologies involving the LMCA field of interventional cardiology, IVUS technology has such as spontaneous or iatrogenic dissection. The incidence progressed in the last two decades. Dedicated training of spontaneous dissection in the LMCA has been reported centers in combination with enthusiasm from a new to be ~1% of all epicardial coronary arteries (6,7). Similar generation of cardiologists complemented by well- to aortic dissection, a spontaneous dissection of the established evidence for simplicity, safety and efficacy of LMCA leads to generation of a false lumen and intramural IVUS systems have led to increased routine use of this hematoma with or without intimal tear that may propagate imaging modality. Currently available catheters use sound retrograde into the aorta. -

Crucial Role of Carotid Ultrasound for the Rapid Diagnosis Of
m e d i c i n a 5 2 ( 2 0 1 6 ) 3 7 8 – 3 8 8 Available online at www.sciencedirect.com ScienceDirect journal homepage: http://www.elsevier.com/locate/medici Clinical Case Report Crucial role of carotid ultrasound for the rapid diagnosis of hyperacute aortic dissection complicated by cerebral infarction: A case report and literature review a a, b a Eglė Sukockienė , Kristina Laučkaitė *, Antanas Jankauskas , Dalia Mickevičienė , a a c a Giedrė Jurkevičienė , Antanas Vaitkus , Edgaras Stankevičius , Kęstutis Petrikonis , a Daiva Rastenytė a Department of Neurology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania b Department of Radiology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania c Institute of Physiology and Pharmacology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania a r t i c l e i n f o a b s t r a c t Article history: Aortic dissection is a life-threatening rare condition that may virtually present by any organ Received 24 January 2016 system dysfunction, the nervous system included. Acute cerebral infarction among multiple Received in revised form other neurological and non-neurological presentations is part of this acute aortic syndrome. 14 September 2016 Rapid and correct diagnosis is of extreme importance keeping in mind the possibility of Accepted 8 November 2016 thrombolytic treatment if a patient with a suspected ischemic stroke arrives to the Emergency Available online 19 November 2016 Department within a 4.5-h window after symptom onset. Systemic intravenous thrombolysis in the case of an acute brain infarction due to aortic dissection may lead to fatal outcomes. -

Acute Chest Pain-Suspected Aortic Dissection
Revised 2021 American College of Radiology ACR Appropriateness Criteria® Suspected Acute Aortic Syndrome Variant 1: Acute chest pain; suspected acute aortic syndrome. Procedure Appropriateness Category Relative Radiation Level US echocardiography transesophageal Usually Appropriate O Radiography chest Usually Appropriate ☢ MRA chest abdomen pelvis without and with Usually Appropriate IV contrast O MRA chest without and with IV contrast Usually Appropriate O CT chest with IV contrast Usually Appropriate ☢☢☢ CT chest without and with IV contrast Usually Appropriate ☢☢☢ CTA chest with IV contrast Usually Appropriate ☢☢☢ CTA chest abdomen pelvis with IV contrast Usually Appropriate ☢☢☢☢☢ US echocardiography transthoracic resting May Be Appropriate O Aortography chest May Be Appropriate ☢☢☢ MRA chest abdomen pelvis without IV May Be Appropriate contrast O MRA chest without IV contrast May Be Appropriate O MRI chest abdomen pelvis without IV May Be Appropriate contrast O CT chest without IV contrast May Be Appropriate ☢☢☢ CTA coronary arteries with IV contrast May Be Appropriate ☢☢☢ MRI chest abdomen pelvis without and with Usually Not Appropriate IV contrast O ACR Appropriateness Criteria® 1 Suspected Acute Aortic Syndrome SUSPECTED ACUTE AORTIC SYNDROME Expert Panel on Cardiac Imaging: Gregory A. Kicska, MD, PhDa; Lynne M. Hurwitz Koweek, MDb; Brian B. Ghoshhajra, MD, MBAc; Garth M. Beache, MDd; Richard K.J. Brown, MDe; Andrew M. Davis, MD, MPHf; Joe Y. Hsu, MDg; Faisal Khosa, MD, MBAh; Seth J. Kligerman, MDi; Diana Litmanovich, MDj; Bruce M. Lo, MD, RDMS, MBAk; Christopher D. Maroules, MDl; Nandini M. Meyersohn, MDm; Saurabh Rajpal, MDn; Todd C. Villines, MDo; Samuel Wann, MDp; Suhny Abbara, MD.q Summary of Literature Review Introduction/Background Acute aortic syndrome (AAS) includes the entities of acute aortic dissection (AD), intramural hematoma (IMH), and penetrating atherosclerotic ulcer (PAU). -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Public Use Data File Documentation
Public Use Data File Documentation Part III - Medical Coding Manual and Short Index National Health Interview Survey, 1995 From the CENTERSFOR DISEASECONTROL AND PREVENTION/NationalCenter for Health Statistics U.S. DEPARTMENTOF HEALTHAND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics CDCCENTERS FOR DlSEASE CONTROL AND PREVENTlON Public Use Data File Documentation Part Ill - Medical Coding Manual and Short Index National Health Interview Survey, 1995 U.S. DEPARTMENT OF HEALTHAND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics Hyattsville, Maryland October 1997 TABLE OF CONTENTS Page SECTION I. INTRODUCTION AND ORIENTATION GUIDES A. Brief Description of the Health Interview Survey ............. .............. 1 B. Importance of the Medical Coding ...................... .............. 1 C. Codes Used (described briefly) ......................... .............. 2 D. Appendix III ...................................... .............. 2 E, The Short Index .................................... .............. 2 F. Abbreviations and References ......................... .............. 3 G. Training Preliminary to Coding ......................... .............. 4 SECTION II. CLASSES OF CHRONIC AND ACUTE CONDITIONS A. General Rules ................................................... 6 B. When to Assign “1” (Chronic) ........................................ 6 C. Selected Conditions Coded ” 1” Regardless of Onset ......................... 7 D. When to Assign -

Computed Tomography Angiographic Assessment of Acute Chest Pain
SA-CME ARTICLE Computed Tomography Angiographic Assessment of Acute Chest Pain Matthew M. Miller, MD, PhD,* Carole A. Ridge, FFRRCSI,w and Diana E. Litmanovich, MDz Acute chest pain leads to 6 million Emergency Depart- Abstract: Acute chest pain is a leading cause of Emergency Depart- ment visits per year in the United States.1 Evaluation of acute ment visits. Computed tomography angiography plays a vital diag- chest pain often leads to a prolonged inpatient assessment, nostic role in such cases, but there are several common challenges with assessment duration often exceeding 12 hours. The associated with the imaging of acute chest pain, which, if unrecog- estimated cost of a negative inpatient chest pain assessment nized, can lead to an inconclusive or incorrect diagnosis. These 2,3 imaging challenges fall broadly into 3 categories: (1) image acquis- amounts to $8 billion per year in the United States. ition, (2) image interpretation (including physiological and pathologic The main challenge to diagnosis is the broad range of mimics), and (3) result communication. The aims of this review are to pathologies that can cause chest pain. Vascular causes describe and illustrate the most common challenges in the imaging of include pulmonary embolism (PE), traumatic and acute chest pain and to provide solutions that will facilitate accurate spontaneous aortic syndromes including aortic transection, diagnosis of the causes of acute chest pain in the emergency setting. dissection, intramural hematoma, and penetrating athero- sclerotic ulcer, aortitis, and coronary artery disease. The Key Words: acute chest pain, challenges, pulmonary angiography, latter will not be discussed in detail because of the com- aortography, computed tomography plexity and breadth of this topic alone. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography. -

Final Addenda FY 2005
FY 2005 Final Addenda ICD-9-CM Volume 3, Procedures Effective October 1, 2004 Tabular List 00.0 Therapeutic ultrasound Add exclusion term Excludes: diagnostic ultrasound (non-invasive) (88.71-88.79) intracardiac echocardiography [ICE] (heart chamber(s)) (37.28) intravascular imaging (adjunctive) (00.21-00.29) New code 00.16 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Ex-vivo treatment of vessel Hyperbaric pressurized graft [conduit] New code 00.17 Infusion of vasopressor agent New subcategory 00.2 Intravascular imaging of blood vessels Endovascular ultrasonography Intravascular ultrasound (IVUS) Code also any synchronous diagnostic or therapeutic procedures Excludes: therapeutic ultrasound (00.01-00.09) New code 00.21 Intravascular imaging of extracranial cerebral vessels Common carotid vessels and branches Intravascular ultrasound (IVUS), extracranial cerebral vessels Excludes: diagnostic ultrasound (non-invasive) of head and neck (88.71) New code 00.22 Intravascular imaging of intrathoracic vessels Aorta and aortic arch Intravascular ultrasound (IVUS), intrathoracic vessels Vena cava (superior) (inferior) Excludes: diagnostic ultrasound (non-invasive) of other sites of thorax (88.73) New code 00.23 Intravascular imaging of peripheral vessels Imaging of: vessels of arm(s) vessels of leg(s) Intravascular ultrasound (IVUS), peripheral vessels Excludes: diagnostic ultrasound (non-invasive) of peripheral vascular system (88.77) New code 00.24 Intravascular imaging of coronary vessels Intravascular