Clinical Guideline Optical Coherence Tomography (OCT)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fluorescein Angiography Findings in Both Eyes of a Unilateral Retinoblastoma Case During Intra-Arterial Chemotherapy with Melphalan
Int J Ophthalmol, Vol. 12, No. 12, Dec.18, 2019 www.ijo.cn Tel: 8629-82245172 8629-82210956 Email: [email protected] ·Letter to the Editor· Fluorescein angiography findings in both eyes of a unilateral retinoblastoma case during intra-arterial chemotherapy with melphalan Cem Ozgonul1, Neeraj Chaudhary2, Raymond Hutchinson3, Steven M. Archer1, Hakan Demirci1 1Department of Ophthalmology and Visual Sciences, W.K. was inserted into the left femoral artery, advanced into the Kellogg Eye Center, MI 48105, USA internal carotid and up to the origin of the ophthalmic artery. 2Department of Radiology, University of Michigan, MI 48109, Once the catheter tip position was confirmed at the origin USA of the ophthalmic artery by fluoroscopy, 5 mg melphalan 3Department of Pediatric Hematology/Oncology, University of was infused in a pulsatile fashion over 30min. There was Michigan, MI 48109, USA no anatomical variant of orbital vascular structure. During Correspondence to: Hakan Demirci. Department of the 2nd IAC, following the infusion of melphalan, sodium Ophthalmology and Visual Science, W.K. Kellogg Eye Center, fluorescein dye at a dose of 7.7 mg/kg was injected through the 1000 Wall St, Ann Arbor, MI 48105, USA. hdemirci@med. same microcatheter. Real-time FA was recorded by using the umich.edu RetCam III (Clarity Medical Systems, Pleasanton, California). Received: 2018-11-01 Accepted: 2019-04-09 FA was repeated 4wk later during the 3rd IAC in the same manner, before infusion of the chemotherapy. In both sessions, DOI:10.18240/ijo.2019.12.24 there was no catheterization or injection of contrast material into the untreated carotid and ophthalmic artery. -

Risk Factors for Adverse Reactions of Fundus Fluorescein Angiography
Original Article Risk factors for adverse reactions of fundus fluorescein angiography Yi Yang1, Jingzhuang Mai2, Jun Wang1 1Department of Ophthalmology, 2Epidemiology Division, Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong General Hospital, Guangzhou 510080, China Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) Provision of study materials or patients: Y Yang; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: Y Yang, JZ Mai; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Yi Yang. Department of Ophthalmology, Guangdong General Hospital, #106, Zhongshan Second Road, Guangzhou 510080, China. Email: [email protected]. Background: To explore the difference between the outcomes of correlations between a series of variables and adverse reactions (ARs) to fluorescein from univariate and multivariate analysis and to evaluate the nausea effects in different age groups. Methods: A retrospective study of patients undergoing consecutive fluorescein angiography between March 2010 and February 2012 was conducted. No patients were excluded on the ground of age, presence of atopy, allergy history, previous procedures without severe allergic ARs, asymptomatic hypertension and kidney failure with serum creatinine levels lower than 250 μmol/L or with renal dialysis. Results: A total of 829 patients were enrolled and 22.2% of them had ARs. The majority of reactions were nausea (12.1%) which occurred less when age became old (P<0.0001). When the correlations between a series of variables and ARs were assessed separately, age (P<0.0001), prior reactions (P<0.0001) and motion sickness (P=0.0062) were highly and cardio/cerebrovascular disease (P=0.0015), diabetes (P=0.0001) and renal disease (P=0.0219) were lowly related to ARs. -

National Correct Coding Initiative's (Ncci) General
NATIONAL CORRECT CODING INITIATIVE’S (NCCI) GENERAL CORRESPONDENCE LANGUAGE AND SECTION-SPECIFIC EXAMPLES (FOR NCCI PROCEDURE TO PROCEDURE (PTP) EDITS AND MEDICALLY UNLIKELY EDITS (MUE)) EFFECTIVE: April 1, 2017* *INCLUDES 2017 HCPCS/CPT CODES Current Procedural Terminology (CPT) codes, descriptions and other data only are copyright 2016 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association. Applicable FARS\DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for the data contained or not contained herein. TABLE OF CONTENTS Section Page Introduction 5 General Correspondence Language for NCCI PTP Edits and Medically Unlikely Edits (MUEs) Standard preparation/monitoring services for anesthesia 8 HCPCS/CPT procedure code definition 8 CPT Manual or CMS manual coding instruction 8 Mutually exclusive procedures 9 Sequential procedure 9 CPT “Separate procedure” definition 9 More extensive procedure 9 Gender-specific procedures 10 Standards of medical/surgical practice 10 Anesthesia service included in surgical procedure 10 Laboratory panel 10 Deleted/modified edits for NCCI 11 Misuse of column two code with column one code 11 Medically Unlikely Edits (MUE) (Units of Service) 11 Deleted/modified edits -

2Nd Quarter 2001 Medicare Part a Bulletin
In This Issue... From the Intermediary Medical Director Medical Review Progressive Corrective Action ......................................................................... 3 General Information Medical Review Process Revision to Medical Record Requests ................................................ 5 General Coverage New CLIA Waived Tests ............................................................................................................. 8 Outpatient Hospital Services Correction to the Outpatient Services Fee Schedule ................................................................. 9 Skilled Nursing Facility Services Fee Schedule and Consolidated Billing for Skilled Nursing Facility (SNF) Services ............. 12 Fraud and Abuse Justice Recovers Record $1.5 Billion in Fraud Payments - Highest Ever for One Year Period ........................................................................................... 20 Bulletin Medical Policies Use of the American Medical Association’s (AMA’s) Current Procedural Terminology (CPT) Codes on Contractors’ Web Sites ................................................................................. 21 Outpatient Prospective Payment System January 2001 Update: Coding Information for Hospital Outpatient Prospective Payment System (OPPS) ......................................................................................................................... 93 he Medicare A Bulletin Providers Will Be Asked to Register Tshould be shared with all to Receive Medicare Bulletins and health care -

Bilateral Exudative Retinal Detachment in a Patient with Cerebral Venous Sinus Thrombosis: a Case Report
Bilateral exudative retinal detachment in a patient with cerebral venous sinus thrombosis: a case report Liang Li The second Xiangya Hospital, Central South University Ling Gao ( [email protected] ) Second Xiangya Hospital https://orcid.org/0000-0002-9850-2038 Case report Keywords: exudative retinal detachment, cerebral venous sinus thrombosis, elschnig spot, retinal capillary ischemia Posted Date: June 6th, 2019 DOI: https://doi.org/10.21203/rs.2.9789/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/8 Abstract Background: Cerebral venous sinus thrombosis (CVST) is a rare cerebrovascular disease, it’s ocular symptoms often characterized by a subacute bilateral visual loss, or diplopia and paralysis of eye movements. Fundus examination usually presents as bilateral papilledema and other ocular signs are rare. We report a case of bilateral multiple retinal detachments and nally diagnosed as CVST. Case presentation: A 49-year old woman with progressive headache and bilateral vision deterioration visited our clinic. Ophthaomological examinations including medical history, best-corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy, fundus ophthalmoscopy, uorescein angiography and Optical coherence tomography and head Magnetic Resonance Venogram (MRV) was also performed. Blood tests for ruling out systemic diseases were also performed. Fundus exam revealed bilateral multiple retinal detachment with sub-retinal uid and blurred disc margin. Fluorescein angiography (FA) revealed early hypouorescence in the background stage, multiple pinpoint leakages at the level of retinal pigment epithelium (RPE), and late pooling to outline the boundary of retinal detachment, with some of the leakage shaped as multiple circles in the late stage of FA. -

Introduction
RIMS, IMPHAL ANNUAL REPORT 2014-15 INTRODUCTION 1. DESCRIPTION : The Regional Institute of Medical Sciences (RIMS), Imphal was established in the year 1972. It is an institution of regional importance catering to the needs of the North Eastern Region in the field of imparting undergraduate and post graduate medical education.The Institution brings together educational facilities for the training of personnel in all important branches of medical specialities including Dental and Nursing education in one place. The Institute is affiliated to the Manipur University, Canchipur, Imphal. 2. MANAGEMENT : The Institute was transferred to the Ministry of Health & Family Welfare, Government of India from North Eastern Council, Shillong (under Ministry of DoNER, Government of India) w.e.f. 1st April, 2007. Under the existing administrative set-up, the highest decision making body is the Board of Governors headed by the Union Minister of Health & Family Welfare as the President and the Director of the Institute as the Secretary. The Executive Council is responsible for the management of the Institute. The Secretary, Ministry of Health & Family Welfare, Government of India is the Chairman of the Executive Council while the head of the Institute remains as Secretary. Thus, the institute is managed at two levels, namely the Board of Governors and the Executive Council. A. Board of Governors : 1. Hon’ble Union Minister, - President Health & Family Welfare, Government of India. 2. Hon’ble Chief Minister, Manipur. - Vice-President 3. A Representative of the Planning Commission, - Member Government of India. 4. Health Ministers of the Beneficiary States - Member 5. Secretary, Ministry of Health & Family Welfare, - Member Government of India. -
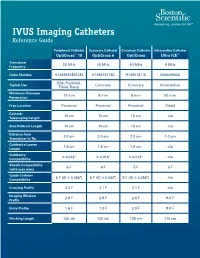
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Fluorescein and Indocyanine Green Angiography Guidelines ______
Fluorescein and Indocyanine Green Angiography Guidelines _______________________________________________________________________________ Approved by: Board Last reviews: 21 January 2012, 3 June 2015 Approval date: 2007 Next review: 3 June 2018 The Royal Australian and New Zealand College of Ophthalmologists ACN 000 644 404 94-98 Chalmers Street, Surry Hills NSW 2010 Phone: +61 2 9690 1001 Fax: +61 2 9690 1321 www.ranzco.edu Introduction and purpose These Guidelines have been issued by RANZCO for the guidance of ophthalmologists. They should not be used by any other persons or provided to patients as a replacement for medical advice. 1. Fluorescein angiography (FA) is an extremely useful and minimally invasive diagnostic investigation that is frequently performed in ophthalmologists’ private practices and ophthalmology departments. Indocyanine Green (ICG) angiography is a similar but less frequently performed investigation. Despite the generally low risks of the procedure, deaths have occurred during and following FA in both Australia and overseas. General 2. Guidelines in eye care are neither minimal nor aspirational but represent quality eye care commensurate with knowledge as at the date of issue. These Guidelines are based on the best available scientific data and on the collective judgement and evaluation of available evidence by retinal specialists in consultation with medico legal and medical (immunology) colleagues. 3. The Guidelines are for the pattern-of-practice rather than the care of a particular individual. While they may meet the needs of most patients, they cannot possibly meet the needs of all patients. 4. Adherence to these Guidelines will not ensure a successful outcome in every situation and the Guidelines should not be deemed inclusive of all proper methods of care or exclusive of other methods of care reasonably directed at obtaining the best results. -

Optos 200Tx and Heidelberg Spectralis
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 19, 2021 Performance evaluation of two fundus oculi angiographic imaging system: Optos 200Tx and Heidelberg Spectralis SHUANG LI, JING‑JING WANG, HONG‑YANG LI, WEI WANG, MENG TIAN, XU‑QIANG LANG and KANG WANG Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, P.R. China Received December 15, 2018; Accepted October 29, 2019 DOI: 10.3892/etm.2020.9451 Abstract. The present study aimed to compare the imaging Introduction performance of two ultra‑wide‑field fluorescein angiog‑ raphy imaging systems, namely the OptosOptomap 200Tx Ultra‑wide‑field fluorescein angiography (UWFA) is a novel (Optos 200Tx) and the Heidelberg Spectralis (Spectralis). A total technology that has developed rapidly in recent years (1,2). of 18 patients (36 eyes) underwent angiography using the two As numerous pathological changes of fundus diseases occur systems at the Department of Ophthalmology, Beijing Friendship at the edge of the retina, the limitation of imaging leads to Hospital (Beijing, China) between January and June 2017. The insufficient diagnosis or underestimation of the severity of the images were obtained as a single shot centered on the macula. disease (3,4). Therefore, clear imaging of the edge of the retina The total area and area within each of four visualized quadrants is important for the diagnosis, monitoring and prognostication were calculated and compared. The averages of the total and of patients with ocular fundus diseases. The traditional fundus individual quadrant area captured by the Optos 200Tx were fluorescein angiography system may only provide a vision field all larger than those obtained with the Spectralis (P<0.05). -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Indocyanine Green Angiography
British Journal of Ophthalmology 1996; 80: 263-266 263 PERSPECTIVE Br J Ophthalmol: first published as 10.1136/bjo.80.3.263 on 1 March 1996. Downloaded from Indocyanine green angiography Sarah L Owens A brief survey of the recent ophthalmic literature under- initial investigations of ICG was published by Flower and scores a resurgence of interest in indocyanine green angio- Hochheimer, as well as by others.4-9 Some of their initial graphy (ICGA). This imaging method may be particularly work described a camera adapted with appropriate exciter useful for the ophthalmologist, as it provides at least a and barrier filters such that simultaneous ICG and fluores- theoretical advantage for improved imaging of the choroidal cein angiography could be performed after a single injec- circulation when compared with fluorescein angiography. tion of a combined mixture of ICG and fluorescein dyes.6 New temporal and spatial technological advances (that is, The ICG dosage used in these initial absorption angio- videoangiography and scanning laser ophthalmoscopy, res- graphy studies tended to be similar to the amount used for pectively) are primarily responsible for this renewed interest. cardiac flow studies - that is, 2 mg per kilogram body Thus, ICGA could become instrumental in elucidating the weight.' The early clinical investigators were uniformly pathogenetic mechanisms of a variety of diseases processes, disappointed in the lack of usefulness of ICGA in the con- as well as serving as a diagnostic and therapeutic tool. firmation of suspected choroidal neovascularisation.9-11 The flurry of recent publications on ICGA, particularly ICGA was not a useful guide to laser therapy of choroidal regarding age-related maculopathy (ARM), suggests a new neovascularisation, but appeared to show particular standard of care for the management of patients with this promise in evaluation and monitoring growth of choroidal disorder. -

Fluorescein Angiography: Preventing and Responding to Complications
Fluorescein Angiography: Preventing and Responding to Complications Jacqueline G. Jaffee, J.D. OMIC Risk Management Specialist PURPOSE OF RISK MANAGEMENT RECOMMENDATIONS OMIC regularly analyzes its claims experience to determine loss prevention measures that our insured ophthalmologists can take to reduce the likelihood of professional liability lawsuits. OMIC policyholders are not required to implement these risk management recommendations. Use your professional judgment in determining the applicability of a given recommendation to their particular patients and practice situation. These loss prevention documents may refer to clinical care guidelines such as the American Academy of Ophthalmology’s Preferred Practice Patterns, peer-reviewed articles, or to federal or state laws and regulations. However, our risk management recommendations do not constitute the standard of care nor do they provide legal advice. If legal advice is desired or needed, consult an attorney. Information contained here is not intended to be a modification of the terms and conditions of the OMIC professional and limited office premises liability insurance policy. Please refer to the OMIC policy for these terms and conditions. Version 1/24/08 Fluorescein angiography (FA) is a diagnostic procedure in which a rapid sequence of photographs is taken to document the blood circulation of the retina/choroid. The dye is usually injected into a vein in the arm, forearm, or hand. While generally well tolerated, angiography is an invasive procedure with associated risks, very rarely but notably of a life-threatening allergic reaction. The following risk management recommendations have been compiled to assist you and your staff so that you may both prevent and better respond to the risks of the procedure.