Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acr–Nasci–Sir–Spr Practice Parameter for the Performance and Interpretation of Body Computed Tomography Angiography (Cta)
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice parameters and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice parameter and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review and approval. The practice parameters and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice parameter and technical standard by those entities not providing these services is not authorized. Revised 2021 (Resolution 47)* ACR–NASCI–SIR–SPR PRACTICE PARAMETER FOR THE PERFORMANCE AND INTERPRETATION OF BODY COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA) PREAMBLE This document is an educational tool designed to assist practitioners in providing appropriate radiologic care for patients. Practice Parameters and Technical Standards are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care1. -

Miniature Integrated Optical Coherence Tomography (OCT) - Ultrasound (US) Probe for Intravascular Imaging
Miniature integrated optical coherence tomography (OCT) - ultrasound (US) probe for intravascular imaging Jiawen Lia, Jiechen Yina, Xiang Lib, Joe Jingc, David Mukaic, Sari Mahonc, Ahmad Edrisd, Khiet Hoangd, K. Kirk Shungb, Matthew Brennerce, Jagat Narulad, Qifa Zhoub, Patel, Pranavd and Zhongping Chenac a Department of Biomedical Engineering, University of California, Irvine, Irvine, California 92697 b NIH Ultrasonic Transducer Resource Center and Department of Biomedical Engineering, University of Southern California, Los Angeles, California 90089 c Beckman Laser Institute, University of California, Irvine, 1002 Health Sciences Road East, Irvine, California 92612 d Division of Cardiology, University of California, Irvine Medical Center, Orange, California 92868 e Division of Pulmonary and Critical Care, University of California, Irvine Medical Center, Orange, California 92868 SUMMARY A miniature integrated optical coherence tomography (OCT) – ultrasound (US) probing system for real-time intravascular imaging has been developed. The outer diameter of the integrated probe is 0.69 mm, which is small enough for imaging in human coronary arteries. This probe, which has high resolution and deep tissue penetration, is designed to identify vulnerable atherosclerotic plaques in coronary arteries. The first in vivo images of a rabbit abdominal aorta obtained by the integrated OCT-US probe are presented. Keywords: atherosclerosis, vulnerable plaque, intravascular ultrasound, optical coherent tomography ABSTRACT Coronary artery atherosclerosis is a major public health problem associated with high clinical morbidity and mortality. For over 20 years, intravascular ultrasound (IVUS) imaging has been a standard diagnostic tool for atherosclerosis1. Recently, optical coherence tomography (OCT) with high resolution, has been applied to intravascular imaging because it enables direct imaging of thin fibrous cap2, one of the key feature of vulnerable atherosclerotic plaque, and tissue responses to stent implantation1. -
Final Program
FinalProgram:2007 2/15/07 11:54 AM Page 1 American Institute of Ultrasound in Medicine Final Program American Institute of Ultrasound in Medicine 14750 Sweitzer Ln, Suite 100 Laurel, MD 20707-5906 USA 301-498-4100 • 800-638-5352 Fax: 301-498-4450 www.aium.org FinalProgram:2007 2/15/07 11:55 AM Page 2 The new ACUSON Antares. Do it all. In creating the new ACUSON Antares™ ultrasound system, premium edition, we investigated your latest, most pressing clinical issues. The result — a system designed around you, enabling you to excel at virtually any ultrasound challenge. Across the full range of ultrasound examinations, including cardiac imaging, the new Antares system delivers the impressive combination of superior image quality, operator-friendly ErgoDynamic™ imaging system design, applications versatility, and the latest advancements in clinical workflow. So no matter what diagnostic challenge walks in the door, you’ll know you already have the answer. 1-888-826-9702 www.usa.siemens.com/medical A91112-61172-A1-4A00 © 2007 Siemens Medical Solutions USA, Inc. All rights reserved. FinalProgram:2007 2/15/07 11:55 AM Page 3 Table of Contents Schedule at a Glance.....................................1 General Information .....................................3 Educational Opportunities ...........................5 Committee Meeting Schedule .......................6 Professional Interest Section Meeting Schedule.......................................7 CME Credit Information...............................8 Faculty Disclosures.......................................9 -

Cardiovascular Research Scholarship Program
2021 CARDIOVASCULAR RESEARCH SCHOLARSHIP PROGRAM - THE 24TH TRANS-PACIFIC RESEARCH OPPORTUNITY - Stanford University The Cardiovascular Medicine Division at Stanford University features diverse faculty and provides opportunities for research in many cardiovascular subspecialties. Stanford’s Center for Cardiovascular Technology (CCVT) facilities include animal laboratories, device development programs, and participation in multi-center cardiology trials, which are supported by Core Angiographic and Intravascular Ultrasound Laboratories. Visiting Scholars to Stanford’s CCVT represent many countries and a variety of research disciplines, resulting in a stimulating and productive research environment. URL: http://med.stanford.edu/ccvt Stanford Scholarship Program Oce c/o: Abbott Medical Japan LLC, VASCULAR Div. Attention: Stanford Scholarship Program TEL: 03-4560-0780 FAX: 03-4560-0781 E-mail: [email protected] Address: Sumitomo Fudosan Mita Twin Building West 3-5-27 Mita, Minato, Tokyo, 108-6304, Japan URL: www.cardiovascular.abbott/jp/ja/hcp/resources/ education-training/stanford.html * Abbott Medical Japan LLC, as a management representative, will jointly use the personal information disclosed in your application form with Stanford University Medical Center in the selection process for this study program and the enrollment procedures to Stanford University Medical Center. © 2020 Abbott. All rights reserved. (APJ00000928-JPN-Rev.A) MEDICINE Stanford Center for Cardiovascular Technology Stanford University School of Medicine Introduction Stanford Center for Cardiovascular Technology Stanford University School of Medicine The Stanford Center for Cardiovascular Technology, formally known as the Center for Research in Cardiovascular Interventions founded at Stanford in 1994, is a core facility for development and testing of new devices in cardiovascular medicine. The Center focuses on early-stage concepts for new technologies, providing a clearinghouse where these ideas can be refined and tested in animal models and clinical studies. -

Equilibrium Radionuclide Angiography/ Multigated Acquisition
EQUILIBRIUM RADIONUCLIDE ANGIOGRAPHY/ MULTIGATED ACQUISITION Equilibrium Radionuclide Angiography/ Multigated Acquisition S van Eeckhoudt, Bravis ziekenhuis, Roosendaal VJR Schelfhout, Rijnstate, Arnhem 1. Introduction Equilibrium radionuclide angiography (ERNA), also known as radionuclide ventriculography (ERNV), gated synchronized angiography (GSA), blood pool scintigraphy or multi gated acquisition (MUGA), is a well-validated technique to accurately determine cardiac function. In oncology its high reproducibility and low inter observer variability allow for surveillance of cardiac function in patients receiving potentially cardiotoxic anti-cancer treatment. In cardiology it is mostly used for diagnosis and prognosis of patients with heart failure and other heart diseases. 2. Methodology This guideline is based on available scientifi c literature on the subject, the previous guideline (Aanbevelingen Nucleaire Geneeskunde 2007), international guidelines from EANM and/or SNMMI if available and applicable to the Dutch situation. 3. Indications Several Class I (conditions for which there is evidence and/or general agreement that a given procedure or treatment is useful and effective) indications exist: • Evaluation of left ventricular function in cardiac disease: - Coronary artery disease - Valvular heart disease - Congenital heart disease - Congestive heart failure • Evaluation of left ventricular function in non-cardiac disease: - Monitoring potential cardiotoxic side effects of (chemo)therapy - Pre-operative risk stratifi cation in high risk surgery • Evaluation of right ventricular function: - Congenital heart disease - Mitral valve insuffi ciency - Heart-lung transplantation 4. Contraindications None 5. Medical information necessary for planning • Clear description of the indication (left and/or right ventricle) • Previous history of cardiac disease • Previous or current use of cardiotoxic medication PART I - 211 Deel I_C.indd 211 27-12-16 14:15 EQUILIBRIUM RADIONUCLIDE ANGIOGRAPHY/ MULTIGATED ACQUISITION 6. -

Comparison of Echocardiography and Angiography in Determining the Cause of Severe Aortic Regurgitation
Br Heart J: first published as 10.1136/hrt.51.1.36 on 1 January 1984. Downloaded from Br Heart J 1984; 51: 36-45 Comparison of echocardiography and angiography in determining the cause of severe aortic regurgitation NICHOLAS L DEPACE, PASQUALE F NESTICO, MORRIS N KOTLER, GARY S MINTZ, DEMETRIOS KIMBIRIS, INDER P GOEL, E ELAINE GLAZIER-LASKEY, JOHN ROSS From the LikoffCardiovascular Institute, Hahnemann University, Philadelphia, Pennsylvania, USA SUMMARY To assess the accuracy of echocardiography in determining the cause of aortic regurgita- tion M mode and cross sectional echocardiography were compared with angiography in 43 patients with predominant aortic regurgitation. Each patient had all three investigations performed during the same admission to hospital. In each instance, the cause of aortic regurgitation was confirmed at surgery or necropsy. Seventeen patients had rheumatic aortic valve disease, 13 bacterial endocarditis with a perforated or partially destroyed cusp, five a biscuspid aortic valve (four with a history of endocarditis), and eight aortic regurgitation secondary to aortic root dilatation or aneurysm. Overall sensitivity of echocardiography and aortography was 84% in determining the cause of aortic regurgi- tation. Thus, rheumatic valve disease and endocarditis appear to be the most common causes of severe aortic regurgitation in this hospital based population. Furthermore, echocardiography is a sensitive non-invasive technique for determining the cause of aortic regurgitation and allows differentiation of valvular from root causes of aortic regurgitation. Aortic regurgitation may be caused by valvular dis- ment for predominant aortic regurgitation were http://heart.bmj.com/ ease, aortic root disease, or a combination of both. reviewed. -

Training a Team of Skilled Operators
Training a Team of Skilled Operators Jeffrey W. Moses, MD John and Myrna Daniels Professor of Cardiology Director, Interventional Cardiac Therapeutics Columbia University Medical Center Director Complex Coronary Interventions St. Francis Hospital, Roslyn, LI Disclosure Statement of Financial Interest I, Jeffrey W. Moses, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context of the subject of this presentation. Perspective on PCI in the Past Decade • Coronary population in the cath lab suffered precipitous decline • A philosophy of “watchful waiting” (i.e., let’s let things get really bad first) otherwise known as GDMT holds sway Algorithm for Guideline-Directed Medical Therapy for Patients With SIHD* (cont.) * Colors correspond to the ACCF/AHA Classification of Recommendations and Levels of Evidence Table. The Basic CHIP Premise • There is a large underserved patient population that can benefit from revascularization Rather than focusing on low-risk patients who may be “easy to treat”, we need to focus upon higher-risk patients who have the most to gain These patients will be more commonly seen as our field / the healthcare system evolves The development of comprehensive specialists trained with advanced technical and cognitive skills to assess and treat these patients is clearly needed Cardiogenic Shock or Arrest* *SCAI Shock Categories: B-E E Inpatient Hospital Complex Comorbidity** or Complex PCI*** **Complex comorbidity: -

Clinical Use of Intracoronary Imaging. Part 2: Acute Coronary Syndromes
CORONARY INTERVENTIONS Euro CLINICAL RESEARCH Intervention 2019;15: 434-451 434-451 published online online published Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous June 2019 Cardiovascular Interventions 3 Thomas W. Johnson/RUHQ]5lEHU, Carlo di Mario , Christos Bourantas+DLER-LD, Alessio Mattesini, Nieves Gonzalo-RVH0GHOD7RUUH+HUQDQGH], Francesco Prati8, Konstantinos Koskinas0LFKDHO-RQHU9, Maria D. Radu, David Erlinge, Evelyn Regar, Vijay Kunadian, Akiko Maehara, Robert A. Byrne9, Davide Capodanno, Takashi Akasaka, WilliamWijns, Gary S. Mintz, Giulio Guagliumi* The authors’ affiliations can be found in the Appendix paragraph. Endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter Endocardiovascular Therapeutics (HKSTENT) and the Cardiac Society of Australia and New Zealand This paper also includes supplementary data published online at: https://eurointervention.pcronline.com/doi/10.4244/EIJY19M06_02 This consensus document is the second of two reports summarizing the views of an expert panel organ- KEYWORDS ized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) on the clinical use of intracoronary imaging including intravascular ultrasound (IVUS), optical coherence tomography • percutaneous (OCT), and near infrared spectroscopy (NIRS)-IVUS. Beyond guidance of stent selection and optimization coronary -
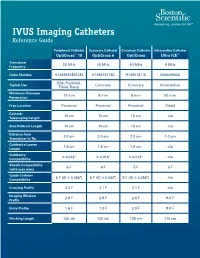
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Intravascular Endoscopy Improvement Through Narrow-Band Imaging
Int J CARS DOI 10.1007/s11548-017-1579-4 ORIGINAL ARTICLE Intravascular endoscopy improvement through narrow-band imaging Axel Boese1 · Akhil Karthasseril Sivankutty1 · Alfredo Illanes1 · Michael Friebe1 Received: 11 January 2017 / Accepted: 21 March 2017 © The Author(s) 2017. This article is an open access publication Abstract Purpose Purpose Recent advances in endoscopy have led to new technologies with significant optical imaging improvements. Through technology advancements and the introduction Since its development a few years ago, narrow-band imaging of complete new methodologies, endoscopic intravascu- (NBI) has already been proved useful in detecting malignant lar visualisation is becoming more and more interesting lesions and carcinoma in clinical settings of urology, gas- as a diagnostic tool. Intravascular imaging could be used troenterology and ENT. The potential of this technology for to identify conditions that lead to strokes, aneurysm and imaging applications of the arterial vessel wall has not been embolism. In vivo imaging of the arteries could addition- properly analysed yet, but with the observed benefits could ally help to find calcifications [1], plaques [2,3], thrombus prove valuable for this clinical use as well. [4] and also to assess the placement of stents [5]. Thus Methods In order to assess the efficacy of NBI, defects such intravascular imaging could play an important role in the as burns and mechanical tears were created on the walls of an clinical decision making process and provide therapy rele- arterial vessel sample. Ex vivo imaging using NBI and white vant information. Several studies have been conducted using light imaging (WLI) were performed with rigid and flexible X-ray-based angioscopic systems to illustrate and record fibre endoscopes. -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Measurement of Peak Rates of Left Ventricular Wall Movement in Man Comparison of Echocardiography with Angiography
British HeartJournal, I975, 37, 677-683. Br Heart J: first published as 10.1136/hrt.37.7.677 on 1 July 1975. Downloaded from Measurement of peak rates of left ventricular wall movement in man Comparison of echocardiography with angiography D. G. Gibson and D. J. Brown From the Cardiac Department, Brompton Hospital, London, and the Medical Computer Centre, Westminster Hospital, London Estimates ofpeak systolic and diastolic rates of left ventricular wall movement were made in 23 patients by echocardiography and angiocardiography. Echocardiographic measurements were calculated as the rate of change of the transverse left ventricular dimension, derived continuously throughout the cardiac cycle. These were compared with similar plots of transverse left ventricular diameter, in the same patients, derived from digitized cineangiograms taken within IO minutes of echocardiograms. The results indicate close correlation between the two methods, and suggest that either can be used to measure peak rates of left ventricular wall movements in patients with heart disease. Identification of echoes arising from the interven- Echocardiograms tricular septum and posterior wall of the left In order to reduce the time interval between the two ventricle has proved to be a significant advance in investigations, echocardiograms were performed at the study of cardiac function by allowing the trans- cardiac catheterization using techniques that have pre- verse diameter of the left ventricle to be measured viously been described (Gibson, 1973). Clear, con- http://heart.bmj.com/ at end-systole and end-diastole (Chapelle and tinuous echoes were obtained from the posterior surface Mensch, I969; Feigenbaum et al., I969). More of the septum and the endocardium ofthe posterior wall recently, it has been possible to derive this dimension of the left ventricle, which were distinguished from those originating from the mitral valve apparatus.