Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Miniature Integrated Optical Coherence Tomography (OCT) - Ultrasound (US) Probe for Intravascular Imaging
Miniature integrated optical coherence tomography (OCT) - ultrasound (US) probe for intravascular imaging Jiawen Lia, Jiechen Yina, Xiang Lib, Joe Jingc, David Mukaic, Sari Mahonc, Ahmad Edrisd, Khiet Hoangd, K. Kirk Shungb, Matthew Brennerce, Jagat Narulad, Qifa Zhoub, Patel, Pranavd and Zhongping Chenac a Department of Biomedical Engineering, University of California, Irvine, Irvine, California 92697 b NIH Ultrasonic Transducer Resource Center and Department of Biomedical Engineering, University of Southern California, Los Angeles, California 90089 c Beckman Laser Institute, University of California, Irvine, 1002 Health Sciences Road East, Irvine, California 92612 d Division of Cardiology, University of California, Irvine Medical Center, Orange, California 92868 e Division of Pulmonary and Critical Care, University of California, Irvine Medical Center, Orange, California 92868 SUMMARY A miniature integrated optical coherence tomography (OCT) – ultrasound (US) probing system for real-time intravascular imaging has been developed. The outer diameter of the integrated probe is 0.69 mm, which is small enough for imaging in human coronary arteries. This probe, which has high resolution and deep tissue penetration, is designed to identify vulnerable atherosclerotic plaques in coronary arteries. The first in vivo images of a rabbit abdominal aorta obtained by the integrated OCT-US probe are presented. Keywords: atherosclerosis, vulnerable plaque, intravascular ultrasound, optical coherent tomography ABSTRACT Coronary artery atherosclerosis is a major public health problem associated with high clinical morbidity and mortality. For over 20 years, intravascular ultrasound (IVUS) imaging has been a standard diagnostic tool for atherosclerosis1. Recently, optical coherence tomography (OCT) with high resolution, has been applied to intravascular imaging because it enables direct imaging of thin fibrous cap2, one of the key feature of vulnerable atherosclerotic plaque, and tissue responses to stent implantation1. -
Final Program
FinalProgram:2007 2/15/07 11:54 AM Page 1 American Institute of Ultrasound in Medicine Final Program American Institute of Ultrasound in Medicine 14750 Sweitzer Ln, Suite 100 Laurel, MD 20707-5906 USA 301-498-4100 • 800-638-5352 Fax: 301-498-4450 www.aium.org FinalProgram:2007 2/15/07 11:55 AM Page 2 The new ACUSON Antares. Do it all. In creating the new ACUSON Antares™ ultrasound system, premium edition, we investigated your latest, most pressing clinical issues. The result — a system designed around you, enabling you to excel at virtually any ultrasound challenge. Across the full range of ultrasound examinations, including cardiac imaging, the new Antares system delivers the impressive combination of superior image quality, operator-friendly ErgoDynamic™ imaging system design, applications versatility, and the latest advancements in clinical workflow. So no matter what diagnostic challenge walks in the door, you’ll know you already have the answer. 1-888-826-9702 www.usa.siemens.com/medical A91112-61172-A1-4A00 © 2007 Siemens Medical Solutions USA, Inc. All rights reserved. FinalProgram:2007 2/15/07 11:55 AM Page 3 Table of Contents Schedule at a Glance.....................................1 General Information .....................................3 Educational Opportunities ...........................5 Committee Meeting Schedule .......................6 Professional Interest Section Meeting Schedule.......................................7 CME Credit Information...............................8 Faculty Disclosures.......................................9 -

Cardiovascular Research Scholarship Program
2021 CARDIOVASCULAR RESEARCH SCHOLARSHIP PROGRAM - THE 24TH TRANS-PACIFIC RESEARCH OPPORTUNITY - Stanford University The Cardiovascular Medicine Division at Stanford University features diverse faculty and provides opportunities for research in many cardiovascular subspecialties. Stanford’s Center for Cardiovascular Technology (CCVT) facilities include animal laboratories, device development programs, and participation in multi-center cardiology trials, which are supported by Core Angiographic and Intravascular Ultrasound Laboratories. Visiting Scholars to Stanford’s CCVT represent many countries and a variety of research disciplines, resulting in a stimulating and productive research environment. URL: http://med.stanford.edu/ccvt Stanford Scholarship Program Oce c/o: Abbott Medical Japan LLC, VASCULAR Div. Attention: Stanford Scholarship Program TEL: 03-4560-0780 FAX: 03-4560-0781 E-mail: [email protected] Address: Sumitomo Fudosan Mita Twin Building West 3-5-27 Mita, Minato, Tokyo, 108-6304, Japan URL: www.cardiovascular.abbott/jp/ja/hcp/resources/ education-training/stanford.html * Abbott Medical Japan LLC, as a management representative, will jointly use the personal information disclosed in your application form with Stanford University Medical Center in the selection process for this study program and the enrollment procedures to Stanford University Medical Center. © 2020 Abbott. All rights reserved. (APJ00000928-JPN-Rev.A) MEDICINE Stanford Center for Cardiovascular Technology Stanford University School of Medicine Introduction Stanford Center for Cardiovascular Technology Stanford University School of Medicine The Stanford Center for Cardiovascular Technology, formally known as the Center for Research in Cardiovascular Interventions founded at Stanford in 1994, is a core facility for development and testing of new devices in cardiovascular medicine. The Center focuses on early-stage concepts for new technologies, providing a clearinghouse where these ideas can be refined and tested in animal models and clinical studies. -

Training a Team of Skilled Operators
Training a Team of Skilled Operators Jeffrey W. Moses, MD John and Myrna Daniels Professor of Cardiology Director, Interventional Cardiac Therapeutics Columbia University Medical Center Director Complex Coronary Interventions St. Francis Hospital, Roslyn, LI Disclosure Statement of Financial Interest I, Jeffrey W. Moses, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context of the subject of this presentation. Perspective on PCI in the Past Decade • Coronary population in the cath lab suffered precipitous decline • A philosophy of “watchful waiting” (i.e., let’s let things get really bad first) otherwise known as GDMT holds sway Algorithm for Guideline-Directed Medical Therapy for Patients With SIHD* (cont.) * Colors correspond to the ACCF/AHA Classification of Recommendations and Levels of Evidence Table. The Basic CHIP Premise • There is a large underserved patient population that can benefit from revascularization Rather than focusing on low-risk patients who may be “easy to treat”, we need to focus upon higher-risk patients who have the most to gain These patients will be more commonly seen as our field / the healthcare system evolves The development of comprehensive specialists trained with advanced technical and cognitive skills to assess and treat these patients is clearly needed Cardiogenic Shock or Arrest* *SCAI Shock Categories: B-E E Inpatient Hospital Complex Comorbidity** or Complex PCI*** **Complex comorbidity: -

Clinical Use of Intracoronary Imaging. Part 2: Acute Coronary Syndromes
CORONARY INTERVENTIONS Euro CLINICAL RESEARCH Intervention 2019;15: 434-451 434-451 published online online published Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous June 2019 Cardiovascular Interventions 3 Thomas W. Johnson/RUHQ]5lEHU, Carlo di Mario , Christos Bourantas+DLER-LD, Alessio Mattesini, Nieves Gonzalo-RVH0GHOD7RUUH+HUQDQGH], Francesco Prati8, Konstantinos Koskinas0LFKDHO-RQHU9, Maria D. Radu, David Erlinge, Evelyn Regar, Vijay Kunadian, Akiko Maehara, Robert A. Byrne9, Davide Capodanno, Takashi Akasaka, WilliamWijns, Gary S. Mintz, Giulio Guagliumi* The authors’ affiliations can be found in the Appendix paragraph. Endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter Endocardiovascular Therapeutics (HKSTENT) and the Cardiac Society of Australia and New Zealand This paper also includes supplementary data published online at: https://eurointervention.pcronline.com/doi/10.4244/EIJY19M06_02 This consensus document is the second of two reports summarizing the views of an expert panel organ- KEYWORDS ized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) on the clinical use of intracoronary imaging including intravascular ultrasound (IVUS), optical coherence tomography • percutaneous (OCT), and near infrared spectroscopy (NIRS)-IVUS. Beyond guidance of stent selection and optimization coronary -
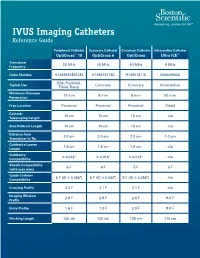
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Intravascular Endoscopy Improvement Through Narrow-Band Imaging
Int J CARS DOI 10.1007/s11548-017-1579-4 ORIGINAL ARTICLE Intravascular endoscopy improvement through narrow-band imaging Axel Boese1 · Akhil Karthasseril Sivankutty1 · Alfredo Illanes1 · Michael Friebe1 Received: 11 January 2017 / Accepted: 21 March 2017 © The Author(s) 2017. This article is an open access publication Abstract Purpose Purpose Recent advances in endoscopy have led to new technologies with significant optical imaging improvements. Through technology advancements and the introduction Since its development a few years ago, narrow-band imaging of complete new methodologies, endoscopic intravascu- (NBI) has already been proved useful in detecting malignant lar visualisation is becoming more and more interesting lesions and carcinoma in clinical settings of urology, gas- as a diagnostic tool. Intravascular imaging could be used troenterology and ENT. The potential of this technology for to identify conditions that lead to strokes, aneurysm and imaging applications of the arterial vessel wall has not been embolism. In vivo imaging of the arteries could addition- properly analysed yet, but with the observed benefits could ally help to find calcifications [1], plaques [2,3], thrombus prove valuable for this clinical use as well. [4] and also to assess the placement of stents [5]. Thus Methods In order to assess the efficacy of NBI, defects such intravascular imaging could play an important role in the as burns and mechanical tears were created on the walls of an clinical decision making process and provide therapy rele- arterial vessel sample. Ex vivo imaging using NBI and white vant information. Several studies have been conducted using light imaging (WLI) were performed with rigid and flexible X-ray-based angioscopic systems to illustrate and record fibre endoscopes. -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Transplant Coronary Heart Disease: Challenges and Solutions
Transplant Research and Risk Management Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Transplant coronary heart disease: challenges and solutions Jacob C Jentzer1 Abstract: Cardiac allograft vasculopathy (CAV) remains one of the leading causes of death and Gavin W Hickey1 graft failure after heart transplantation. A variety of causes, including donor heart characteristics, Sameer J Khandhar2,3 recipient risk factors, and immune-mediated influences, are associated with developing CAV. In this review, we will focus on the pathophysiology of developing CAV and various methods 1Heart and Vascular Institute, University of Pittsburgh Medical to screen for this condition. The pathogenesis of CAV likely involves repeated injuries to the Center, Pittsburgh, PA, USA; 2Perelman endothelium from a variety of factors such as cellular-mediated rejection, and alloimmune School of Medicine, University factors, including antibody-mediated injury, ischemia-reperfusion injury at time of transplant, of Pennsylvania, Philadelphia, PA, USA; 3Heart and Vascular Institute, cytomegalovirus infections, immunosuppression medications, systemic inflammation, and tra- Penn-Presbyterian Medical Center, ditional atherosclerosis risk factors. Patients with significant CAV are often asymptomatic, and Philadelphia, PA, USA therefore early detection by routine screening prior to graft dysfunction is crucial. There are a variety of invasive, noninvasive, and blood tests that have been studied as screening methods, and we will discuss the role of each of these in this review article. Although some treatment regimens have been established for CAV, this is an area where further studies and research are necessary. Keywords: cardiac allograft vasculopathy, orthotopic heart transplantation, intra-vascular imaging Introduction Cardiac allograft vasculopathy (CAV) is widely considered the “Achilles heel” of heart transplantation.1 Early posttransplant survival has improved, but median survival remains only 10.4 years. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Intravascular Ultrasound Imaging: a New Method for Guiding Interventional Vascular Procedures
UCLA UCLA Previously Published Works Title Intravascular ultrasound imaging: a new method for guiding interventional vascular procedures. Permalink https://escholarship.org/uc/item/43b8t0wq Journal Echocardiography (Mount Kisco, N.Y.), 7(4) ISSN 0742-2822 Authors Tobis, JM Mallery, JA Mahon, D et al. Publication Date 1990-07-01 DOI 10.1111/j.1540-8175.1990.tb00382.x License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Intravascular Ultrasound Imaging: A New Method for Guiding Interventional Vascular Procedures JONATHAN M. TOBIS, M.D., JOHN A. MALLERY, M.D., DONALD MAHON, M.D., JAMES GRIFFITH, PH.D., JIM GESSERT, B.A., KENNETH LEHMANN, M.D., and WALTER L. HENRY, M.D. Cardiology Division, University of California, Irvine; Cardiology Division, Veterans Administration Hospital, Long Beach; and InterTherapy, Inc., Costa Mesa, California A wide variety of therapeutic procedures have terie~.~-~Such external ultrasound devices have been developed to intervene in patients with obvious limitations for evaluating human coro- coronary artery disease. Whether the interven- nary arteries in vivo. An intravascular ultra- tion is intended to increase lumen size by dilat- sound transducer placed on the end of a catheter ing the artery or to decrease atheroma mass by has been used in vitro and in vivo animal studies laser ablation, mechanical atherectomy, or by to image coronary and peripheral arteries.'-'' lowering serum cholesterol, it will be important This approach allows the arterial wall to be to quantitate atherosclerotic plaque volume be- imaged in cross section from inside the artery, fore and after treatment. -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography.