Intravascular Ultrasound-Guided Interventions in Coronary Artery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Office Address
11/21/2013 CURRICULUM VITAE Barry T. Katzen, M.D., FACR, FACC, FSIR OFFICE ADDRESS: Founder and Medical Director Baptist Cardiac & Vascular Institute Baptist Hospital of Miami 8900 North Kendall Drive Miami, Florida 33176 (786) 596-7050 PERSONAL INFORMATION: Marital Status: Married Children: Three EDUCATION: College: Emory University 1963-1964 Atlanta, Georgia University of Miami 1964-1966 Miami, Florida Medical School: University of Miami School of Medicine 1966-1970 Miami, Florida (early admission) Doctor of Medicine June 4, 1970 Internship: Jackson Memorial Hospital Miami, Florida (Straight Medicine) 6/24/70~6/24/71 Radiology Residency: The New York Hospital-Cornell Medical Center 1971-1974 Including: Memorial Hospital; Sloan-Kettering Cancer Institute; Hospital for Special Surgery Fellowship: St. Vincents Hospital/Medical Center Cardiovascular Radiology New York, New York 1974-1975 11/21/2013 FOREIGN STUDIES: Angiography Sept.-Nov. 1974 Policlinico Umberto Primo Instituto di Radiologia “La Sapiemza” University of Rome, Italy Plinio Rossi, M.D., Director Pulmonary Radiology and Chest Special Procedures Sept.-Oct. 1973 The Brompton Chest Hospital London, England Ian Kerr, M.D., Director FOREIGN STUDIES: Chest Pathology June-Aug. 1969 National Institute of Health Grant The Brompton Chest Hospital London, England Lynne Reid, M.D., Director LICENSURE: New York (National Boards) 1971 Florida 1970 Virginia 1976 District of Colombia 1976 CERTIFICATION: • The American Board of Radiology ~ 6/23/74 • The National Board of Medical Examiners ~ -

Andreas Gruentzig — the Life and Death of a Pioneer
Folia Cardiol. 2006, Vol. 13, No. 4, pp. 348–350 Copyright © 2006 Via Medica PEARLS AND GIANTS IN CARDIOLOGY ISSN 1507–4145 Andreas Gruentzig — the life and death of a pioneer The history of cardiology is rich The innovation of placing a latex with pioneers whose contributions pa- balloon in an angiographic catheter be- ved the way to the advancement of in- longs to Dr. Portman, and this trigge- vasive cardiology, but not all of them red Dr. Gruentzig to use this method have been recognized and appreciated to dilate a constricted coronary artery. for their innovations and accomplish- It took Andreas two years to solve “mi- ments. The late Andreas Gruentzig nor” technical problems from conce- was a pioneer, who was acclaimed even iving his idea till its true application. It in his lifetime, as were Forssmann, is now legendary that Gruentzig wor- Cournand and Richards, who were even ked, together with his wife Michaela, crowned by the Nobel Prize Commit- his assistant Maria Schlumpf and her tee. There were many other pioneers husband Walter, in the family kitchen whose contributions are less recognized in history, in the evenings trying many versions of the balloon like Otto Klein of Prague, while other outstanding cli- catheter. The preparations included, among other nicians whose contributions were widely known even things, cooperation with factories, chemists and during their life time, but in retrospect it seems that many other technical experts. After hundreds of unfortunately did not receive enough official recogni- experiments, Gruentzig was satisfied with the tech- tion as in the case of Charles T. -

St Luke's Cardiac History Timeline with Photos, 1903-2003
Advocate Aurora Health Advocate Aurora Health Institutional Repository Aurora St. Luke’s Medical Center Books, Documents, and Pamphlets Aurora St. Luke’s Medical Center November 2017 St Luke's Cardiac History Timeline with Photos, 1903-2003 Aurora Health Care Follow this and additional works at: https://institutionalrepository.aah.org/aslmc_books This Book is brought to you for free and open access by the Aurora St. Luke’s Medical Center at Advocate Aurora Health Institutional Repository. It has been accepted for inclusion in Aurora St. Luke’s Medical Center Books, Documents, and Pamphlets by an authorized administrator of Advocate Aurora Health Institutional Repository. For more information, please contact [email protected]. 19 03—1959 The Wright Brothers America the Beautiful z_— Ford Motor Company Picture it . America in 1903. z The west was won and the * economy was flourishing. Our nation was truly coming into its own. 1903 telephone %iJvaikee Nilie S t 0 ii C S The nations hiqenufty, hidustry and we&th teuched Mflwaukee n I 90% and as a result, akied in buikfln the cornertone.s o Harley-Davidson St. Luke’s Medical Center’s story begins A Legacy is Born in 1903 with humble roots and a vision for caring for the medical needs of Milwaukee. William F. Malone, MD. added an office wing to his castle-like mansion on the corner of Madison and Hanover (now 3rd Street) and opened Malone Hospital, the first hospital on the city’s south side. Dt Malone served as the medical director and head of surgery for the next 15 years. -

Coronary Drug- Eluting Stents
Updated BRS Data: Safety Issues and Long-Term Benefit Seo Suk Min Seoul St. Mary’s Hospital Cardiovascular Center 1 PCI and DES have revolutionized cardiovascular care 1977 1988 2001 - 2003 Andreas Gruentzig perfor Julio Palmaz and Richard Schatz de Drug-eluting stents are introduc ms the first PTCA in Zurich velop a stainless steel stent for cor ed to the European and U.S. ma , Switzerland onary applications rkets Balloon Angio Bare Coronary Drug- plasty1977 (PTCA) Metal Stents ( Eluting Stents BMS) (DES) • Continuous PCI technology advancements improved clinical outcomes for CAD • BVS represents a new approach transient vessel support with drug delivery capability without the long-term limitations of the metallic drug-eluting stents (DES) *Additional sizes received CE Mark in August 2012. 2 2 Potential Fate of Vessels Stented with DESs Delayed Healing Stent Thrombosis? * uncovered struts1 Benign, low grade, Non-occlusive Neo-Atheroma Stent Thrombosis? In-Stent Restenosis (symptomatic or asymptomatic) Late Acquired Malapposition Stent Thrombosis? 3 Information contained herein for distribution outside the U.S. only. AP2940435-OUS Rev. A 09/14 Despite improvements in PCI, there is evidence of unmet need with current treatment options Long-term BMS event rate1 Long-term DES event rate2 1. Yamaji K, et al. Very long-term (15 to 20 years) clinical and angiographic outcome after coronary bare metal stent implantation. Circ Cardiovasc Interv 2010;3(5):468-75. 2. SPIRIT III: Ischemia-driven TLR through 5 years. Stone GW, TCT 2011. 4 4 Three Approaches to Improve Early and Late DES Outcomes 1. Metallic DES with bioabsorbable polymers 2. -
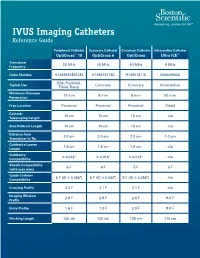
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Viewed by Members of Our 8
COVER STORY Multivessel Stenting in the Current DES Era A case report and discussion. BY ANDREAS WALI, MD, FACC, FSCAI ntil the recent concerns about late and very late of randomized trials comparing bare-metal stents to coro- thrombosis with drug-eluting stents (DESs), the nary artery bypass grafts were underway, including ERACI II advent of these devices was greeted with great and ARTS I for multivessel coronary artery disease.18-21 These enthusiasm as one of the final tools needed for studies did not reveal any mortality benefit, and there was a Uunparalleled long-term success with percutaneous revascu- narrowing of clinical outcomes to 14% in ARTS I compared larization. The RAVEL trial,1 utilizing a sirolimus-eluting to the pre-stent CABRI. stent, revealed no in-stent restenosis in relatively simple Not long after these studies were available came news of lesions. The subsequent SIRIUS trials with sirolimus-eluting DESs with the promise of abolishing the Achilles’ heel of stents and the numerous TAXUS trials with the paclitaxel- angioplasty: restenosis. The debate continues, with surgeons eluting stents revealed marked and consistent reductions in noting that previous trials looked at highly selected patients endpoints in increasingly complex lesions.2-8 The use of at relatively low risk and preserved left ventricular function, these devices in allcomers as studied in the RESEARCH and who were not representative of the types of patients who T-SEARCH registries by Dr. Patrick W. Serruys, and the per- usually undergo surgery, and with interventionists arguing sonal experiences of interventionists with DESs, have led to that with DESs, the whole equation has changed. -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Multi-Vessel Coronary Disease and Percutaneous
Coronary disease MULTI-VESSEL CORONARY DISEASE Heart: first published as 10.1136/hrt.2003.018986 on 13 February 2004. Downloaded from AND PERCUTANEOUS CORONARY INTERVENTION 341 Cash Casey, David P Faxon Heart 2004;90:341–346. doi: 10.1136/hrt.2003.018986 he goal of percutaneous coronary intervention (PCI) is to provide a safe, effective, less invasive alternative to coronary artery bypass graft surgery (CABG). When introduced by TAndreas Gruentzig 25 years ago, he envisioned the procedure to be a technique that would delay the need for CABG until severe multi-vessel coronary disease was present. Over the years, technological advances in equipment and devices have improved safety as well as short and long term outcomes. This has greatly expanded the indications for the technique and allowed more arteries to be accessible to effective treatment with better patient outcomes. In addition, developments in adjuvant pharmacotherapy have further improved outcomes of percutaneous procedures. The results of many large trials in the 1990s have shown that percutaneous intervention can be equally successful when compared to the ‘‘gold standard’’ CABG for patients with multi-vessel coronary artery disease. Now with advances in coronary stent technology, including drug eluting stents, multi-vessel angioplasty is set to make another leap forward with further expansion of the indications and improved outcomes. Approximately two thirds of patients who require revascularisation have multi-vessel disease and two thirds of these have anatomy that is amenable to treatment by percutaneous or open heart procedures.1 Both techniques have been shown to be relatively safe and highly effective in relieving angina, and have similar mortality and myocardial infarction rates; however, all the major studies have shown fewer additional revascularisation procedures in patients who undergo open heart surgery.1 It is widely anticipated that the gap in repeat procedures may begin to close with the advent of drug eluting stents. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Pulse of CRF the Newsletter of the Cardiovascular Research Foundation Winter 2013 - Vol 7, TCT Issue
Pulse of CRF The Newsletter of the Cardiovascular Research Foundation Winter 2013 - Vol 7, TCT Issue IN THIS ISSUE Cutting-Edge Clinical Research Presented at TCT 2013 ....................................... 1 CRF Selects Jack Lewin, MD, to Serve as President and CEO ........................ 1 In Memoriam: Andreas Gruentzig, MD, Receives TCT Career Achievement Award ..................... 2 TCT Goes Tablet ............................. 3 Cutting-Edge Clinical Research Presented at TCT 2013 The annual Transcatheter substantially reducing the need for ongoing and has enrolled nearly Cardiovascular Therapeutics (TCT) printed materials. Highlights from double the number of patients scientific symposium is the world’s the scientific sessions included: included in the current research. preeminent forum for interventional New TAVR Options on Further, the REPRISE II trial tested cardiologists, cardiac surgeons, and the safety of a second-generation vascular medicine specialists. the Horizon: TAVR device internationally. The The pivotal CoreValve Extreme Risk TCT celebrated a milestone 25 years Lotus Valve System was associated trial found that transcatheter aortic at the 2013 meeting held in San with low rates of complications in valve replacement (TAVR) with Francisco, California. Attracting over symptomatic patients with severe the CoreValve device substantially 11,500 attendees, the symposium aortic blockages who were at reduced the incidence of death broke new ground by distributing high risk for surgery. Successful and major stroke at 1 year -

Ehrs and Malpractice
A vision of tomorrow: Honoring patient choice BY RICHARD A. SZUCS, MD RRAMIFICATIONS Richard A. Szucs, MD, is a radiologist with Commonwealth Radiology, P.C., and president FALL 2013 n VOLUME 19 n NO. 4 WWW.RAMDOCS.ORG of the Board of Trustees of the Richmond Academy of Medicine. EHRs and tarting a conversation about end-of-life care can be dif- malpractice ficult, whether we are physi- BY CHIP JONES cians, patients, family mem- Secours, HCA and VCU, to advise the participants that the Richmond Sbers, religious and community leaders us regarding how we, working with Academy of Medicine assume a lead- or other professionals. It is, however, others, can promote and encourage ership role as catalyst, convener and imperative that these conversations advance care planning (ACP) in this organizer of a community-wide effort take place. And, once they occur, it is community. The core group agreed on advance care planning. Everyone equally critical that caregivers honor that adopting a uniform approach agrees that advance care planning is a patients’ choices. When no conversa- to ACP across the healthcare mar- lifelong process, best begun before a tion occurs, families and caregivers ketplace was essential for increasing crisis develops. are left making decisions that may awareness and engagement. We shared the outcomes of the As a physician, any discussion of not reflect what a patient desires. In late May 2013, the Acad- conference with health systems, electronic records should start with two The Richmond Academy of emy sponsored a community-wide Secretary of Health and Human words: “audit trail.” Here’s why: Medicine champions advance care educational conference where 100 Resources Bill Hazel, Senator Mark According to the July 2013 issue of the Virginia Medical Law Report, lawyers planning, hospice and palliative care. -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography.