(MRA) and Magnetic Resonance Venography (MRV) Medical Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deep Venous Thrombosis with Suspected Pulmonary Embolism
Thoracic Imaging Peter A. Loud, MD Deep Venous Thrombosis Douglas S. Katz, MD Dennis A. Bruce, MD with Suspected Pulmonary Donald L. Klippenstein, MD Zachary D. Grossman, MD Embolism: Detection with Combined CT Venography Index terms: Computed tomography (CT), 1 angiography, 9*.129142, and Pulmonary Angiography 9*.12915, 9*.12916 Embolism, pulmonary, 60.72 Pulmonary angiography, 944.12914, 944.12915, 944.12916 PURPOSE: To determine the frequency and location of deep venous thrombosis at Veins, thrombosis, 9*.751, 9*.12914 computed tomographic (CT) venography after CT pulmonary angiography in a large series of patients clinically suspected of having pulmonary embolism and to Radiology 2001; 219:498–502 compare the accuracy of CT venography with lower-extremity venous sonography. Abbreviation: MATERIALS AND METHODS: Venous phase images were acquired from the DVT ϭ deep venous thrombosis diaphragm to the upper calves after completion of CT pulmonary angiography in 650 patients (373 women, 277 men; age range, 18–99 years; mean age, 63 years) 1 From the Department of Radiology, to determine the presence and location of deep venous thrombosis. Results of CT Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY 14263 (P.A.L., venography were compared with those of bilateral lower-extremity venous sonog- D.L.K., Z.D.G.), and the Department raphy in 308 patients. of Radiology, Winthrop University Hospital, Mineola, NY (D.S.K., D.A.B.). RESULTS: A total of 116 patients had pulmonary embolism and/or deep venous From the 1999 RSNA scientific assem- thrombosis, including 27 patients with pulmonary embolism alone, 31 patients with bly. -

Spring Programme 2011
Faculty of Radiologists Royal College of Surgeons in Ireland Combined Spring Meeting 8th & 9 th April 2011 Venue: Castlemartyr Hotel, Co. Cork. Programme Faculty of Radiologists, Royal College of Surgeons in Ireland CPD Credits Awarded: 5 Royal College of Radiologists credits are applied for. Friday 8 th April 2011 3.30-4.30pm Registration 4.30-5.30pm Stroke in 2011, Moderator: Dr. Ian Kelly, Waterford Regional Hospital 4.30-5.00pm Acute Stoke Imaging. Dr Noel Fanning, Cork University Hospital, Cork 5.00-5.30pm Stroke: A clinical perspective. Dr. George Pope, John Radcliffe Hospitals, Oxford 5.30-6.30pm Moderator: Dr. Adrian Brady, Dean, Faculty of Radiologists Belfast to Bosnia and Autopsy to Virtopsy Dr. Jack Crane, State Pathologist, NI 8pm Dinner Saturday 9 th April 2011 8.30-9.00am Registration 9.00-10.00am Liver hour. Moderator: Dr John Feeney, AMNCH, Dublin 9.00-9.30am Liver imaging pre metastatectomy. Dr. Peter MacEneaney, Mercy University Hospital, Cork 9.30-10.00am Parenchymal and focal liver biopsy - when and how. Dr Stephen J Skehan St Vincent's University Hospital, Dublin 10.00-11.00am Paediatric Hour. Moderator: Dr. Stephanie Ryan, The Children’s University Hospital Temple Street, Dublin 10.00-10.30am Paediatric Abdominal Emergencies. Dr Eoghan Laffan, The Children’s, University Hospital Temple Street, Dublin 10.30-11.00am Non Accidental Injury. Dr Conor Bogue, Cork University Hospital, Cork 11.00-11.30am Tea/Coffee Break and Poster Exhibition 11.30-12.30pm MSK Hour. Moderator: Dr Orla Buckley, AMNCH, Dublin 11.30-12.00pm Image guided joint interventions. -
CT Venography: Technique and Indications
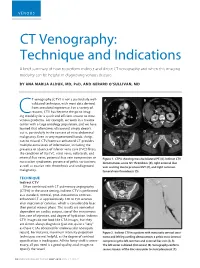
VENOUS CT Venography: Technique and Indications A brief summary of how to perform indirect and direct CT venography and when this imaging modality can be helpful in diagnosing venous disease. BY ANA MARIJA ALDUK, MD, PHD, AND GERARD O’SULLIVAN, MD T venography (CTV) is not a particularly well- A B validated technique, with most data derived from anecdotal experience. For a variety of reasons, CTV has become the go-to imag- Cing modality for a quick and efficient answer to most venous problems. For example, we work in a trauma center with a large oncology population, and we have learned that oftentimes ultrasound simply doesn’t cut it, particularly in the context of intra-abdominal C D malignancy. Even in very experienced hands, things can be missed. CTV/contrast-enhanced CT provides multiple extra levels of information, including the presence or absence of inferior vena cava (IVC) filters; the condition of the IVC, renal veins, collaterals, and internal iliac veins; potential iliac vein compression or Figure 1. CTPA showing massive bilateral PE (A). Indirect CTV nutcracker syndromes; presence of pelvic varicosities; demonstrates acute IVC thrombosis (B), right external iliac as well as ovarian vein thrombosis and undiagnosed vein scarring due to previous DVT (C), and right common malignancy. femoral vein thrombosis (D). TECHNIQUE A C Indirect CTV Often combined with CT pulmonary angiography (CTPA) in the acute setting, indirect CTV is performed as a standard, nonoral, post–intravenous contrast- enhanced CT at approximately 120 to 150 seconds after injection of contrast, which is considerably later than portal venous phase. -

2Nd Quarter 2001 Medicare Part a Bulletin
In This Issue... From the Intermediary Medical Director Medical Review Progressive Corrective Action ......................................................................... 3 General Information Medical Review Process Revision to Medical Record Requests ................................................ 5 General Coverage New CLIA Waived Tests ............................................................................................................. 8 Outpatient Hospital Services Correction to the Outpatient Services Fee Schedule ................................................................. 9 Skilled Nursing Facility Services Fee Schedule and Consolidated Billing for Skilled Nursing Facility (SNF) Services ............. 12 Fraud and Abuse Justice Recovers Record $1.5 Billion in Fraud Payments - Highest Ever for One Year Period ........................................................................................... 20 Bulletin Medical Policies Use of the American Medical Association’s (AMA’s) Current Procedural Terminology (CPT) Codes on Contractors’ Web Sites ................................................................................. 21 Outpatient Prospective Payment System January 2001 Update: Coding Information for Hospital Outpatient Prospective Payment System (OPPS) ......................................................................................................................... 93 he Medicare A Bulletin Providers Will Be Asked to Register Tshould be shared with all to Receive Medicare Bulletins and health care -

Optos 200Tx and Heidelberg Spectralis
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 19, 2021 Performance evaluation of two fundus oculi angiographic imaging system: Optos 200Tx and Heidelberg Spectralis SHUANG LI, JING‑JING WANG, HONG‑YANG LI, WEI WANG, MENG TIAN, XU‑QIANG LANG and KANG WANG Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, P.R. China Received December 15, 2018; Accepted October 29, 2019 DOI: 10.3892/etm.2020.9451 Abstract. The present study aimed to compare the imaging Introduction performance of two ultra‑wide‑field fluorescein angiog‑ raphy imaging systems, namely the OptosOptomap 200Tx Ultra‑wide‑field fluorescein angiography (UWFA) is a novel (Optos 200Tx) and the Heidelberg Spectralis (Spectralis). A total technology that has developed rapidly in recent years (1,2). of 18 patients (36 eyes) underwent angiography using the two As numerous pathological changes of fundus diseases occur systems at the Department of Ophthalmology, Beijing Friendship at the edge of the retina, the limitation of imaging leads to Hospital (Beijing, China) between January and June 2017. The insufficient diagnosis or underestimation of the severity of the images were obtained as a single shot centered on the macula. disease (3,4). Therefore, clear imaging of the edge of the retina The total area and area within each of four visualized quadrants is important for the diagnosis, monitoring and prognostication were calculated and compared. The averages of the total and of patients with ocular fundus diseases. The traditional fundus individual quadrant area captured by the Optos 200Tx were fluorescein angiography system may only provide a vision field all larger than those obtained with the Spectralis (P<0.05). -

Pelvic Venous Reflux Diseases
Open Access Journal of Family Medicine Review Article Pelvic Venous Reflux Diseases Arbid EJ* and Antezana JN Anatomic Considerations South Charlotte General and Vascular Surgery, 10512 Park Road Suite111, Charlotte, USA Each ovary is drained by a plexus forming one major vein *Corresponding author: Elias J. Arbid, South measuring normally 5mm in size. The left ovarian plexus drains into Charlotte General and Vascular Surgery, 10512 Park Road left ovarian vein, which empties into left renal vein; the right ovarian Suite111, Charlotte, NC 28120, USA plexus drains into the right ovarian vein, which drains into the Received: November 19, 2019; Accepted: January 07, anterolateral wall of the inferior vena cava (IVC) just below the right 2020; Published: January 14, 2020 renal vein. An interconnecting plexus of veins drains the ovaries, uterus, vagina, bladder, and rectum (Figure 1). Introduction The lower uterus and vagina drain into the uterine veins and Varicose veins and chronic venous insufficiency are common then into branches of the internal iliac veins; the fundus of the uterus disorders of the venous system in the lower extremities that have drains to either the uterine or the ovarian plexus (utero-ovarian and long been regarded as not worthy of treatment, because procedures salpingo ovarian veins) within the broad ligament. Vulvoperineal to remove them were once perceived as worse than the condition veins drain into the internal pudendal vein, then into the inferior itself. All too frequently, patients are forced to learn to live with them, gluteal vein, then the external pudendal vein, then into the saphenous or find "creative" ways to hide their legs. -

ULTRASOUND STUDY GUIDE • Technical Knowledge O Physics And
ULTRASOUND STUDY GUIDE Technical knowledge o Physics and Safety, understand the following: 1) Physics of sound interactions in the body. 2) How transducers work, how the image is created, and what physical properties are being displayed. 3) Relative strengths and weaknesses of different transducers including various aspects of resolution. Sound properties and interactions Reflection Attenuation Scattering Refraction Absorption Acoustic impedance Speed of sound Wavelength Other . Transducer fundamentals Transmit frequencies Transducer components Transducer types Transducer pros and cons Other . Beam formation Focusing Steering Other . Imaging modes and display 2D 3D 4D Panoramic imaging Compound imaging Harmonic imaging Elastography Contrast imaging Scanning modes o 2D o 3D o 4D o M-mode o Doppler o Other Image orientation Other . Image resolution Axial Lateral Elevational / Azimuthal Temporal Contrast Penetration vs. resolution Other . System Controls - Know the function of the controls listed below and be able to recognize them in the list of scan parameters shown on the image monitor Gain Time gain compensation Power output Focal zone Transmit frequency Depth Width Zoom / Magnification Dynamic range Frame rate Line density Frame averaging / persistence Other . Doppler / Flow imaging – Be familiar with the terminology used to describe Doppler exams. Be able to interpret and optimize the images. Be able to recognize artifacts, know their significance, and know what produces them. Doppler -

Initial Observations Comparing MDCT and 3.0T MRI Findings with Autopsy Findings
Utility of Postmortem Autopsy via Whole- Body Imaging: Initial Observations Comparing MDCT and 3.0T MRI Findings with Autopsy Findings Jang Gyu Cha, MD1 Dong Hun Kim, MD1 Objective: We prospectively compared whole-body multidetector computed Dae Ho Kim, MD2 tomography (MDCT) and 3.0T magnetic resonance (MR) images with autopsy Sang Hyun Paik, MD1 findings. Jai Soung Park, MD1 Materials and Methods: Five cadavers were subjected to whole-body, 16- Seong Jin Park, MD1 channel MDCT and 3.0T MR imaging within two hours before an autopsy. A radi- Hae Kyung Lee, MD1 ologist classified the MDCT and 3.0T MRI findings into major and minor findings, Hyun Sook Hong, MD1 which were compared with autopsy findings. 3 Duek Lin Choi, MD Results: Most of the imaging findings, pertaining to head and neck, heart and 4 Kyung Moo Yang, MD vascular, chest, abdomen, spine, and musculoskeletal lesions, corresponded to 4 Nak Eun Chung, MD autopsy findings. The causes of death that were determined on the bases of 4 Bong Woo Lee, MD MDCT and 3.0T MRI findings were consistent with the autopsy findings in four of 4 Joong Seok Seo, MD five cases. CT was useful in diagnosing fatal hemorrhage and pneumothorax, as well as determining the shapes and characteristics of the fractures and the direc- Index terms: tion of external force. MRI was effective in evaluating and tracing the route of a Computed tomography (CT) metallic object, soft tissue lesions, chronicity of hemorrhage, and bone bruises. Magnetic resonance (MR) Whole-body imaging Conclusion: A postmortem MDCT combined with MRI is a potentially powerful Forensic autopsy tool, providing noninvasive and objective measurements for forensic investiga- DOI:10.3348/kjr.2010.11.4.395 tions. -

503 © Springer Nature Switzerland AG 2021 D. M. Kamat, M. Frei
Index A Acute splenic sequestration crisis (ASSC), 71 Abnormal chromosomal breakage test, 387 Adenosine deaminase activity (ADA), 372 ABO hemolytic disease, 325 Allo-immune thrombocytopenia, 116 Acquired aplastic anemia (AAA) Alternative pathway, 489 clinical features, 371 Anemia, 323, 326 definition and classifications, 371 chronic disease, 375, 376 diagnosis and management, 371 congenital dyserythropoietic anemias, 381 etiology and pathogenesis, 371 fanconi anemia, 374, 375 incidence, 370 folic acid deficiency, 380, 381 Acquired disorders of coagulation iron deficiency anemia, 377–379 coaguloapathy vitamin B12 deficiency, 379, 380 liver failure, 264 ANKRD26-related thrombocytopenia massive transfusion, 264, 265 (ANKRD26-RT), 143 disseminated intravascular Anticoagulation therapy, 348–350 coagulation, 261–263 Antiphospholipid antibodies (APAs), 273 platelet dysfunction, renal failure, 263 Antithrombin (AT) deficiency, 272 sepsis Aplastic anemia (AA) consensus definition, 259 clinical presentation, 393 multiple hematologic diagnosis and severity stratification, manifestations, 259 393, 395 organ injury, 260 differential diagnosis, 393 pathogenesis, 260 eltrombopag, 397 TAMOF, 261 epidemiology, 391 TTP, 261 etiology, 392 Acquired thromboembolic events, 346 hematopoietic stem cell transplant, 398 Acquired von Willebrand Syndrome (AVWS) idiopathic AA, 391 definition, 240 immunosuppressive therapy, 396 diagnosis, 240–242 infection prevention and treatment, 396 management, 242–244 pathophysiology, 392 pathophysiology, 240 transfusion support, -

Polyarteritis Nodosa and Renal Vein Thrombosis: a Case Report and Review of the Literature
International Journal of Case Report Clinical Rheumatology Polyarteritis nodosa and renal vein thrombosis: A case report and review of the literature Renal Vein Thrombosis (RVT) is rare and usually complicates nephrotic syndrome and renal malignancies. Francesco Bozzao*1, We report the case of a 48-year-old woman, who was diagnosed with polyarteritis nodosa (PAN) and Silvano Bettio1, RVT, which was incidentally detected during diagnostic workup. Venous thromboembolism (VTE) can Monica Regis2, 3 complicate the active phases of several vasculitides. Our review of the literature suggests that the risk of Marina Drabeni , Diego Rossi4 & VTE in PAN is lower than that in other vasculitides, although it remains higher during the active disease. 1 Our case reminds clinicians that VTE can be a rare manifestation of PAN. Fabio Fischetti 1Department of Medicine, Azienda Sanitaria Universitaria Integrata and University of Trieste (ASUITs), Strada di Keywords: polyarteritis nodosa • renal vein thrombosis • venous thromboembolism • vasculitis Fiume 449, 34149, Trieste, Italy 2Department of Internal Medicine, Azienda per l'Assistenza Sanitaria n. 2 Bassa Friulana-Isontina, Viale Fatebenefratelli 34, List of Abbreviations: RVT: Renal Vein Thrombosis; HBV: Hepatitis B Virus; DNA: deoxyribonucleic 34170, Gorizia, Italy acid; ANCA: AntiNeutrophil Citoplasmic Antibodies; CT: Computed Tomography; PAN: Polyarteritis 3 Nodosa; VTE: Venous Thromboembolism; AKI: Acute Kidney Injury; AAV: ANCA-Associated Vasculitides; Department of Dermatology, Azienda per l'Assistenza Sanitaria n. 2 Bassa Friulana- BD: Behçet's Disease; GPA: Granulomatosis with Polyangiitis; MPA: Microscopic Polyangiitis; EGPA: Isontina, Viale Fatebenefratelli 34, 34170, Eosinophilic Granulomatosis with Polyangiitis; H&E: Hematoxylin and Eosin Gorizia, Italy 4Department of Gestione Anatomia Patologica, Azienda per l'Assistenza Sanitaria n. -

Unilateral Renal Vein Thrombosis Treated by Nephrectomy and Post-Operative Heparin by E
Arch Dis Child: first published as 10.1136/adc.26.128.358 on 1 August 1951. Downloaded from UNILATERAL RENAL VEIN THROMBOSIS TREATED BY NEPHRECTOMY AND POST-OPERATIVE HEPARIN BY E. W. PARRY From the Paediatric Unit, County Hospital, Bangor (RECEIVED FOR PUBLICATION JANUARY 19, 1951) In the majority of cases renal vein thrombosis is The child's general condition showed him to be pale and secondary to dehydration, sepsis, or both, and has quiet with evidence of dehydration. His chest and heart occurred in enterocolitis, diphtheria, umbilical were normal; his abdomen was normal in appearance and skin infections. It has been and movements. The umbilicus was clean and dry. sepsis, measles, Palpation revealed a large firm mass extending from the recorded as a sequel to pyelo-nephritis due to a level of the costal margin to the iliac crest on the left side. spread from the glomerular to the renal vein. The The mass was perfectly smooth in outline and no notch renal vein may become secondarily involved as a could be felt. It conformed in outline to a renal swelling result of thrombophlebitis in the vena cava, the and could be displaced from the loin. The mass was Protected by copyright. spermatic, or the ovarian veins. This type, however, obviously painful, and any palpation caused marked seems to be confined to adults, and is very rare in distress. No other abnormality could be found on infancy. examination. The right kidney was not palpable. Both sexes are equally involved. The age On rectal examination the mass could be felt in front is in that of cases occur of the rectum at the pelvic brim. -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section