The Mechanism of Low-Temperature Oxidation of Carbon Monoxide by Oxygen Over the Pdcl2–Cucl2/Γ-Al2o3 Nanocatalyst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -
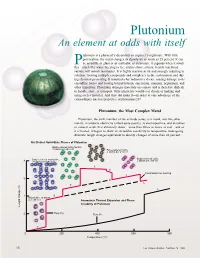
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

STRUCTURAL, ELECTRONIC PROPERTIES and the FEATURES of CHEMICAL BONDING in LAYERED 1111-OXYARSENIDES Larhaso and Lairaso: AB INITIO MODELING
The preprint. Submitted to: Journal of Structural Chemistry STRUCTURAL, ELECTRONIC PROPERTIES AND THE FEATURES OF CHEMICAL BONDING IN LAYERED 1111-OXYARSENIDES LaRhAsO AND LaIrAsO: AB INITIO MODELING V.V. Bannikov, I.R. Shein Institute of Solid State Chemistry, Ural Branch Of Russian Academy of Sciences, 620990, Ekaterinburg, Pervomayskaya St., 91, Russia E-mail: [email protected] The comparative study of structural, electronic properties, topology of the Fermi surface, and the features of chemical bonding in layered 1111-oxyarsenides LaRhAsO and LaIrAsO has been performed based on the results of ab initio modeling of their electronic structure. It was established that only weak sensitivity with respect both to electron and hole doping is expected for LaIrAsO being non-magnetic metal, however, the Rh-containing compound should be characterized with weak band magnetism, and the hole doping is expected to be able to move its ground state away from the boundary of magnetic instability. The mentioned feature allows to consider LaRhAsO oxyarsenide as a possible “electron analogue” of LaFeAsO compound being the initial phase for the layered FeAs-superconductors. Keywords: 1111-phases, oxyarsenides, doping, band structure, Fermi surface, chemical bonding INTRODUCTION The oxychalcogenides and oxypnictides with common chemical formula Me(a)Me(b)(Ch/Pn)O (where Me(a) is atom of Y, La, Bi or 4f-metal, usually taking the oxidation state +3 in compounds, Me(b) is atom of d-metal, Ch = S, Se, Te, Pn = P, As, Sb) form the vast family of so-called layered 1111-phases [1,2] with tetragonal ZrCuSiAs-like structure which can be regarded as the sequence of [Me(a)–O] and [Me(b)–(Ch/Pn)] blocks alternating along the tetragonal axis, where each atom is characterized with coordination number = 4 (Fig.1). -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration. -

List of Oxidation States of the Elements 1
List of oxidation states of the elements 1 List of oxidation states of the elements This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. The most common oxidation states are in bold. This table is based on Greenwood's,[1] with all additions noted. Oxidation state 0, which is found for all elements, is implied by the column with the element's symbol. The format of the table, based on one devised by Mendeleev in 1889, highlights some of the periodic trends. Ä1 H +1 He Li +1 Be +2 Ä1 B +1 +2 +3 [2] Ä4 Ä3 Ä2 Ä1 C +1 +2 +3 +4 Ä3 Ä2 Ä1 N +1 +2 +3 +4 +5 Ä2 Ä1 O +1 +2 Ä1 F Ne Ä1 Na +1 Mg +1 +2 [3] Al +1 +3 Ä4 Ä3 Ä2 Ä1 Si +1 +2 +3 +4 Ä3 Ä2 Ä1 P +1 +2 +3 +4 +5 Ä2 Ä1 S +1 +2 +3 +4 +5 +6 Ä1 Cl +1 +2 +3 +4 +5 +6 +7 Ar Ä1 K +1 Ca +2 Sc +1 +2 +3 Ä1 Ti +2 +3 +4 Ä1 V +1 +2 +3 +4 +5 Ä2 Ä1 Cr +1 +2 +3 +4 +5 +6 Ä3 Ä2 Ä1 Mn +1 +2 +3 +4 +5 +6 +7 Ä2 Ä1 Fe +1 +2 +3 +4 +5 +6 Ä1 Co +1 +2 +3 +4 +5 Ä1 Ni +1 +2 +3 +4 Cu +1 +2 +3 +4 Zn +2 Ga +1 +2 +3 Ä4 Ge +1 +2 +3 +4 Ä3 As +2 +3 +5 List of oxidation states of the elements 2 Ä2 Se +2 +4 +6 Ä1 Br +1 +3 +4 +5 +7 Kr +2 Rb +1 Sr +2 [4] [5] Y +1 +2 +3 Zr +1 +2 +3 +4 Ä1 Nb +2 +3 +4 +5 Ä2 Ä1 Mo +1 +2 +3 +4 +5 +6 Ä3 Ä1 Tc +1 +2 +3 +4 +5 +6 +7 Ä2 Ru +1 +2 +3 +4 +5 +6 +7 +8 Ä1 Rh +1 +2 +3 +4 +5 +6 Pd +2 +4 Ag +1 +2 +3 Cd +2 In +1 +2 +3 Ä4 Sn +2 +4 Ä3 Sb +3 +5 Ä2 Te +2 +4 +5 +6 Ä1 I +1 +3 +5 +7 Xe +2 +4 +6 +8 Cs +1 Ba +2 La +2 +3 Ce +2 +3 +4 Pr +2 +3 +4 Nd +2 +3 Pm +3 Sm +2 +3 Eu +2 +3 Gd +1 +2 +3 Tb +1 +3 +4 Dy +2 +3 Ho +3 Er +3 Tm +2 +3 Yb +2 +3 Lu +3 Hf +2 +3 +4 List of oxidation -

Converting Between the Oxides of Nitrogen Using Metal–
Chem Soc Rev View Article Online TUTORIAL REVIEW View Journal | View Issue Converting between the oxides of nitrogen using metal–ligand coordination complexes Cite this: Chem. Soc. Rev., 2015, 44,6708 Andrew J. Timmons and Mark D. Symes* À À The oxides of nitrogen (chiefly NO, NO3 ,NO2 and N2O) are key components of the natural nitrogen cycle and are intermediates in a range of processes of enormous biological, environmental and industrial importance. Nature has evolved numerous enzymes which handle the conversion of these oxides to/from other small nitrogen-containing species and there also exist a number of heterogeneous catalysts that can mediate similar reactions. In the chemical space between these two extremes exist Received 30th March 2015 metal–ligand coordination complexes that are easier to interrogate than heterogeneous systems and simpler DOI: 10.1039/c5cs00269a in structure than enzymes. In this Tutorial Review, we will examine catalysts for the inter-conversions of the various nitrogen oxides that are based on such complexes, looking in particular at more recent examples www.rsc.org/chemsocrev that take inspiration from the natural systems. Creative Commons Attribution 3.0 Unported Licence. Key learning points (1) The key role of nitrogen oxides (especially nitrite) in the nitrogen cycle. (2) The coordination chemistry in the active sites of the various nitrogen oxide reductase enzymes. (3) How nitrite binds to, and is reduced at, metal ligand coordination complexes. (4) The various platforms that have been used for NO reduction. (5) Nitrate reduction with metal ligand coordination complexes. This article is licensed under a 1. Introduction Open Access Article. -

2013-‐08-‐04 1 Oxidation and Reduction
2013-08-04 Rules for Assigning B) Oxidation State Determination Oxidation State (O.S.)* 1. O.S. of an atom in pure element = 0 Oxidation and Reduction For an atom, electrons can be lost or gained during a 2. O.S. of monoatomic ion = charge chemical reaction. 3. O.S. of Oxygen (O) is -2 in most of its covalent and ionic compounds (-1 in peroxides). If electrons are lost: OXIDATION The state becomes more positive 4. O.S. of H is +1 when combined with a non-metal 5. O.S. of H is -1 when combined with a metal If electrons are gained: REDUCTION The state becomes more negative 6. O.S. of Fluorine (F) is -1 in compounds 7. The sum of O.S. is 0 in compounds Oxidation State (O.S.) or Oxidation Number (O.N.) Number of electrons lost or gained during a chemical 8. The sum of O.S. equals charge in ions change * A compilation from different textbooks Elements with Fixed Oxidation States (O.S.)* Instead of memorizing elements that show different O.S., remember those that have a fixed one: Group 1 (IA) elements: Always +1 Group 2 (IIA) elements: Always +2 Aluminum, Al, and Scandium, Sc: Always +3 Zinc, Zn, and Cadmium, Cd: Always +2 Silver, Ag: Always +1 * A compilation from different textbooks 1 2013-08-04 OXIDATION STATE DETERMINATION EXERCISES Assign oxidation state (O.S.) to each element in the following compounds: a) SO3 i) CrCl3 2- 2- b) SO3 j) CrO4 2- c) KMnO4 k) Cr2O7 d) MnO2 l) HClO4 e) HNO3 m) HClO3 f) S8 n) HClO2 g) CH2Cl2 o) HClO h) SCl2 p) K2SO4 2 2013-08-04 C) A Nomenclature Proposal MORE GENERAL OBSERVATIONS Problematic of Nomenclature Coverage in Textbooks • Flow charts of various complexities (relatively often!) Flow charts, rules, … and tables, tables, tables … (Memorization!) ü From 3-box flow charts to 14-box ones GENERAL OBSERVATIONS • Link with acid/base concepts (sometimes, but rarely!) • Appears in chapters 2 or 3 of textbooks (often!) ü So, it is to be covered “at once”; as a single topic. -

Surface and Corrosion Chemistry of PLUTONIUM
Surface and Corrosion Chemistry of PLUTONIUM John M. Haschke, Thomas H. Allen, and Luis A. Morales s 252 Los Alamos Science Number 26 2000 Surface and Corrosion Chemistry of Plutonium Elemental plutonium, the form in which most of the weapons-grade material exists, is a reactive metal. When exposed to air, moisture, and common elements such as oxygen and hydrogen, the metal surface readily corrodes and forms a powder of small plutonium-containing particles. Being easily airborne and inhaled, these particles pose a much greater risk of dispersal during an accident than the original metal. The present emphasis on enhancing nuclear security through safe mainte- nance of the nuclear stockpile and safe recovery, handling, and storage of surplus plutonium makes it more imperative than ever that the corrosion of plutonium be understood in all its manifestations. etallic plutonium was first a Los Alamos pioneer in plutonium initiated spontaneously at room temper- prepared at Los Alamos in corrosion, was prescient when he ature and advanced into the plutonium M1944, during the Manhattan wrote, “Most investigators are inclined metal at a rate of more than 1 centime- 10 Project. After samples became avail- to concentrate their attention on PuO2, ter per hour (cm/h), or a factor of 10 able, scientists studied the properties which can be well characterized, and faster than the corrosion rate in dry of the metal, including its reactions to ignore other compounds that may air. The reaction generated excessive with air, moisture, oxygen, and hydro- contribute to the overall corrosion temperatures and started under condi- gen. Extensive investigation of pluto- behavior” (Wick 1980). -

Full Field Chemical Imaging of Buried Native Sub-Oxide Layers on Doped Silicon Patterns
Surface Science 604 (2010) 1628–1636 - doi : 10.1016/j.susc.2010.06.006 Published in Elsevier Surface Science Full field chemical imaging of buried native sub-oxide layers on doped silicon patterns a b, a a c F. de la Peña , N. Barrett , L.F. Zagonel , M. Walls , O. Renault a Univ Paris-Sud, Laboratoire de Physique des Solides, CNRS/UMR 8502, Orsay, F-91405, France b CEA, IRAMIS, SPCSI, LENSIS, F-91191 Gif-sur-Yvette, France c CEA-LETI, MINATEC, 17 rue des Martyrs, 38054 Grenoble Cedex 9, France DOI: 10.1016/j.susc.2010.06.006 a b s t r a c t Keywords: Fully energy-filtered X-ray photoelectron emission microscopy is used to analyze the Semiconductor–insulator interfaces spatial distribution of the silicon sub-oxide structure at the SiO2/Si interface as a Semiconductor–semiconductor thin film function of underlying doping pattern. Using a spectroscopic pixel-by-pixel curve fitting structures analysis, we obtain the sub-oxide binding energy and intensity distributions over the full Silicon oxides field of view. Binding energy maps for each oxidation state are obtained with a spatial Photoelectron emission resolution of 120 nm. Within the framework of a five-layer model, the experimental Synchrotron radiation photoelectron data are used to obtain quantitative maps of the sub-oxide layer thickness and also their spectroscopy spatial distribution over the p–n junctions. Variations in the sub-oxide thicknesses are Photoelectron emission microscopy (XPEEM) found to be linked to the level and type of doping. The procedure, which takes into account instrumental artefacts, enables the quantitative analysis of the full 3D dataset. -
Chemistry of the Main Group Elements: Chalcogens Through Noble Gases Sections 8.7-8.10
Chemistry of the Main Group Elements: Chalcogens through Noble Gases Sections 8.7-8.10 Monday, November 9, 2015 Oxygen • Forms compounds with every element except He, Ne, and Ar • Two naturally occurring allotropes: O2 and O3 • O2 has two unpaired electrons and a triplet ground state that moderates its reactivity Oxygen Both O2 and O3 are powerful oxidants remember G nFE Partial reduction of O2 gives hydrogen peroxide Hydrogen peroxide synthesis is achieved with anthraquinone 0 H2/Pd Oxides An oxide is any compound with an oxygen in the 2– oxidation state; there are three types, • basic oxides are formed with metals and give basic solutions when dissolved in water CaO H 2O Ca OH 2 2 MgO 2H Mg H 2O • acidic oxides are formed with p-block elements and give acid solutions when dissolved in water N2O5 H 2O 2HNO3 Sb2O5 2OH 5H 2O 2Sb OH 6 • amphoteric oxides can act as either acids or bases 2 ZnO 2H Zn H 2O 2 ZnO 2OH H 2O Zn OH 4 Sulfur Sulfur has many allotropes • S2, S3, S6, S7, α-S8, β-S8, γ-S8, ... , S20 Considered ‘soft’ compared to oxygen, it bonds strongly to most transition metals μ2-sulfide μ3-sulfide μ4-sulfide Sulfur Halides There are seven different sulfur fluorides... +I +I +II +II 0 +III +I S2F2 SSF2 SF2 FSSF3 +VI +IV +V +V SF4 S2F10 SF6 ...but for the other halogens only S2X2 and SX2 complexes are known S xsCl S Cl xsCl2 SCl 8 2 2 2 FeCl3 2 Halogens Chemistry of the halogens is dominated by the drive to complete the octet.