Structure-Property Relationships of Layered Oxypnictides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -
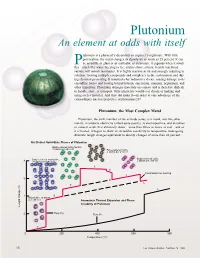
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

Creating New Superconducting & Semiconducting Nanomaterials And
Scholars' Mine Doctoral Dissertations Student Theses and Dissertations Summer 2013 Creating new superconducting & semiconducting nanomaterials and investingating the effect of reduced dimensionality on their properties Sukhada Mishra Follow this and additional works at: https://scholarsmine.mst.edu/doctoral_dissertations Part of the Chemistry Commons Department: Chemistry Recommended Citation Mishra, Sukhada, "Creating new superconducting & semiconducting nanomaterials and investingating the effect of reduced dimensionality on their properties" (2013). Doctoral Dissertations. 2060. https://scholarsmine.mst.edu/doctoral_dissertations/2060 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. CREATING NEW SUPERCONDUCTING & SEMICONDUCTING NANOMATERIALS AND INVESTINGATING THE EFFECT OF REDUCED DIMENSIONALITY ON THEIR PROPERTIES by SUKHADA MISHRA A DISSERTATION Presented to the Faculty of the Graduate School of the MISSOURI UNIVERSITY OF SCIENCE AND TECHNOLOGY In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in CHEMISTRY 2013 Approved by Dr. Manashi Nath, Advisor Dr. Philip Whitefield Dr. Nicholas Leventis Dr. Jeffrey Winiarz Dr. Matthew O’Keefe © 2013 Sukhada Mishra All rights reserved iii PUBLICATION DISSERTATION OPTION This dissertation consists of two published articles, two articles which have been submitted for publication and one manuscript in preparation. Paper I, found on pages 52—88, is published in ‗ACS Nano‘. Paper II included in pages 89—109, has been submitted for publication to ‗Advanced Materials‘. Paper III, found on pages 110—135, is published in ‗Nano Energy‘. -

Synthesis, Structure and Properties of Layered Phosphide Nitrides Akth2mn4p4n2 (Ak = Rb, Cs)
Synthesis, structure and properties of layered phosphide nitrides AkTh2Mn4P4N2 (Ak = Rb, Cs) Bai-Zhuo Li,a Si-Qi Wu,a Zhi-Cheng Wang,a Cao Wang,b and Guang-Han Cao*,a,c a Department of Physics, Zhejiang Province Key Laboratory of Quantum Technology and Devices, Interdisciplinary Center for Quantum Information, and State Key Lab of Silicon Materials, Zhejiang University, Hangzhou 310027, China b School of Physics & Optoelectronic Engineering, Shandong University of Technology, Zibo 255000, China c Collaborative Innovation Centre of Advanced Microstructures, Nanjing University, Nanjing 210093, China Keywords Intercalations | Synthetic methods | Physical properties | Phosphide nitrides | Electronic structures Main observation and conclusion We report the design, synthesis, structure, and properties of two complex layered phosphide nitrides, AkTh2Mn4P4N2 (Ak = Rb, Cs), which contain anti-fluorite-type [Mn2P2] bilayers separated by fluorite-type [Th2N2] layers as a result of the intergrowth between AkMn2P2 and ThMnPN. The new compounds are featured with an intrinsic hole doping associated with the interlayer charge transfer and a built-in chemical pressure from the [Th2N2] layers, both of which are reflected by the changes in the lattice and the atomic position of phosphorus. The measurements of magnetic susceptibility, electrical resistivity, and specific heat indicate existence of local moments as well as itinerant electrons in relation with d-p hybridizations. The expected dominant antiferromagnetic interactions with enhanced d-p hybridizations were demonstrated by the first-principles calculations only when additional Coulomb repulsions are included. The density of states at the Fermi level derived from the specific-heat analysis are 3.5 and 7.5 times of the calculated ones for Ak = Rb and Cs, respectively, suggesting strong electron correlations in the title compounds. -

Download Article (PDF)
Z. Kristallogr. 226 (2011) 435–446 / DOI 10.1524/zkri.2011.1363 435 # by Oldenbourg Wissenschaftsverlag, Mu¨nchen Structural chemistry of superconducting pnictides and pnictide oxides with layered structures Dirk JohrendtI, Hideo HosonoII, Rolf-Dieter HoffmannIII and Rainer Po¨ttgen*, III I Department Chemie und Biochemie, Ludwig-Maximilians-Universita¨t Mu¨nchen, Butenandtstraße 5–13 (Haus D), 81377 Mu¨nchen, Germany II Frontier Research Center, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan III Institut fu¨r Anorganische und Analytische Chemie, Universita¨t Mu¨nster, Corrensstraße 30, 48149 Mu¨nster, Germany Received October 29, 2010; accepted February 6, 2011 Pnictide / Pnictide oxide / Superconductivity / for hydride formation of CeRuSi ! CeRuSiH [6] and Intermetallics / Group-subgroup relation CeRuGe ! CeRuGeH [7]. The crystal chemical data of the huge number of ZrCuSiAs materials have recently Abstract. The basic structural chemistry of supercon- been reviewed [8]. ducting pnictides and pnictide oxides is reviewed. Crystal Although the basic crystallographic data of the many chemical details of selected compounds and group sub- ThCr2Si2 and ZrCuSiAs type compounds are known for group schemes are discussed with respect to phase transi- several years, especially for the ZrCuSiAs family, systema- tions upon charge-density formation, the ordering of va- tic property studies have been performed only recently. cancies, or the ordered displacements of oxygen atoms. These investigations mainly focused on p-type transparent Furthermore, the influences of doping and solid solutions semiconductors like LaCuSO (for a review see [9]) or the on the valence electron concentration are discussed in or- colored phosphide and arsenide oxides REZnPO [10] and der to highlight the structural and electronic flexibility of REZnAsO [11]. -

STRUCTURAL, ELECTRONIC PROPERTIES and the FEATURES of CHEMICAL BONDING in LAYERED 1111-OXYARSENIDES Larhaso and Lairaso: AB INITIO MODELING
The preprint. Submitted to: Journal of Structural Chemistry STRUCTURAL, ELECTRONIC PROPERTIES AND THE FEATURES OF CHEMICAL BONDING IN LAYERED 1111-OXYARSENIDES LaRhAsO AND LaIrAsO: AB INITIO MODELING V.V. Bannikov, I.R. Shein Institute of Solid State Chemistry, Ural Branch Of Russian Academy of Sciences, 620990, Ekaterinburg, Pervomayskaya St., 91, Russia E-mail: [email protected] The comparative study of structural, electronic properties, topology of the Fermi surface, and the features of chemical bonding in layered 1111-oxyarsenides LaRhAsO and LaIrAsO has been performed based on the results of ab initio modeling of their electronic structure. It was established that only weak sensitivity with respect both to electron and hole doping is expected for LaIrAsO being non-magnetic metal, however, the Rh-containing compound should be characterized with weak band magnetism, and the hole doping is expected to be able to move its ground state away from the boundary of magnetic instability. The mentioned feature allows to consider LaRhAsO oxyarsenide as a possible “electron analogue” of LaFeAsO compound being the initial phase for the layered FeAs-superconductors. Keywords: 1111-phases, oxyarsenides, doping, band structure, Fermi surface, chemical bonding INTRODUCTION The oxychalcogenides and oxypnictides with common chemical formula Me(a)Me(b)(Ch/Pn)O (where Me(a) is atom of Y, La, Bi or 4f-metal, usually taking the oxidation state +3 in compounds, Me(b) is atom of d-metal, Ch = S, Se, Te, Pn = P, As, Sb) form the vast family of so-called layered 1111-phases [1,2] with tetragonal ZrCuSiAs-like structure which can be regarded as the sequence of [Me(a)–O] and [Me(b)–(Ch/Pn)] blocks alternating along the tetragonal axis, where each atom is characterized with coordination number = 4 (Fig.1). -

Numerical Studies of Iron Based Superconductors Using Spin- Fermion Models
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2017 Numerical Studies of Iron Based Superconductors using Spin- Fermion Models Christopher Brian Bishop University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Condensed Matter Physics Commons Recommended Citation Bishop, Christopher Brian, "Numerical Studies of Iron Based Superconductors using Spin-Fermion Models. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4735 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Christopher Brian Bishop entitled "Numerical Studies of Iron Based Superconductors using Spin-Fermion Models." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Physics. Adriana Moreo, Major Professor We have read this dissertation and recommend its acceptance: Elbio Dagotto, Steve Johnston, Takeshi Egami Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Numerical Studies of Iron Based Superconductors using Spin-Fermion Models A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Christopher Brian Bishop December 2017 c by Christopher Brian Bishop, 2017 All Rights Reserved. -

1 the Phase Diagrams of Iron-Based Superconductors
The phase diagrams of iron-based superconductors: theory and experiments Les diagrammes de phases des supraconducteurs à base de fer: La théorie etles expériences A. Martinelli1,*, F. Bernardini2,3, S. Massidda3 1 SPIN-CNR, C.so Perrone 24, I-16152 Genova – Italy 2 Dipartimento di Fisica, Università di Cagliari, Cittadella Universitaria, I-09042 Monserrato - Italy 3 IOM-CNR c/o Dipartimento di Fisica, Università di Cagliari, Cittadella Universitaria, I-09042 Monserrato - Italy Abstract Phase diagrams play a primary role in the understanding of materials properties. For iron-based superconductors (Fe-SC), the correct definition of their phase diagrams is crucial because of the close interplay between their crystallo-chemical and magnetic properties, on one side, and the possible coexistence of magnetism and superconductivity, on the other. The two most difficult issues for understanding the Fe-SC phase diagrams are: 1) the origin of the structural transformation taking place during cooling and its relationship with magnetism; 2) the correct description of the region where a crossover between the magnetic and superconducting electronic ground states takes place. Hence a proper and accurate definition of the structural, magnetic and electronic phase boundaries provides an extremely powerful tool for material scientists. For this reason, an exact definition of the thermodynamic phase fields characterizing the different structural and physical properties involved is needed, although it is not easy to obtain in many cases. Moreover, physical properties can often be strongly dependent on the occurrence of micro- structural and other local-scale features (lattice micro-strain, chemical fluctuations, domain walls, grain boundaries, defects), which, as a rule, are not described in a structural phase diagram. -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration. -

The Mechanism of Low-Temperature Oxidation of Carbon Monoxide by Oxygen Over the Pdcl2–Cucl2/Γ-Al2o3 Nanocatalyst
nanomaterials Article The Mechanism of Low-Temperature Oxidation of Carbon Monoxide by Oxygen over the PdCl2–CuCl2/γ-Al2O3 Nanocatalyst Lev Bruk 1, Denis Titov 1, Alexander Ustyugov 1, Yan Zubavichus 2 ID , Valeriya Chernikova 3, Olga Tkachenko 4, Leonid Kustov 4,*, Vadim Murzin 5, Irina Oshanina 1 and Oleg Temkin 1 1 Moscow Technological University, Institute of Fine Chemical Technology, Department of General Chemical Technology, Moscow 119571, Russia; [email protected] (L.B.); [email protected] (D.T.); [email protected] (A.U.); [email protected] (I.O.); [email protected] (O.T.) 2 National Research Centre “Kurchatov Institute”, Moscow 123182, Russia; [email protected] 3 Functional Materials Design, Discovery and Development Research Group (FMD3), Advanced Membranes and Porous Materials Center (AMPMC), Division of Physical Sciences and Engineering (PSE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia; [email protected] 4 N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 119991, Russia; [email protected] 5 Deutsches Elektronen-Synchrotron DESY, D-22607 Hamburg, Germany; [email protected] * Correspondence: [email protected]; Tel.: +7-499-137-2935 Received: 19 February 2018; Accepted: 30 March 2018; Published: 3 April 2018 Abstract: The state of palladium and copper on the surface of the PdCl2–CuCl2/γ-Al2O3 nanocatalyst for the low-temperature oxidation of CO by molecular oxygen was studied by various spectroscopic techniques. Using X-ray absorption spectroscopy (XAS), powder X-ray diffraction (XRD), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), freshly prepared samples of the catalyst were studied. -

Determination of Energy Gap of the Iron-Based
DETERMINATION OF ENERGY GAP OF THE IRON-BASED OXYPNICTIDE AND LaOFeGe SUPERCONDUCTORS USING SPECIFIC HEAT CAPACITY TAREK MOHAMED FAYEZ AHMAD UNIVERSITI SAINS MALAYSIA 2013 DETERMINATION OF ENERGY GAP OF THE IRON-BASED OXYPNICTIDE AND LaOFeGe SUPERCONDUCTORS USING SPECIFIC HEAT CAPACITY By TAREK MOHAMED FAYEZ AHMAD Thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy October 2013 DEDICATION To my father and mother To my brothers and sisters To my wife and my sons ii ACKNOWLEDGMENTS First and foremost, I give praise and thanks to the almighty Allah for giving me the strength to complete my research study. I would like to express my sincerest and deepest gratitude to my fatherly supervisor Professor Kamarulazizi Bin Ibrahim for his wisdom, guidance, and intellectual support. His encouraging attitude and self-less guidance throughout the years have enriched me with empirical insights and knowledge in my scientific and academic career. I would like to thank Universiti Sains Malaysia for their generous financial support. I also thank the staff members of the School of Physics for the co-operation, technical assistance, and support they extended during my studies. Finally, I thank my friends for encouraging and helping me throughout my journey in the academe. Tarek Mohamed Fayez Ahmad Penang, Malaysia. 2013 iii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS iii TABLE OF CONTENTS iv LIST OF TABLES vii LIST OF FIGURES viii LIST OF SYMBOLS xii LIST OF MAJOR ABBREVIATIONS xiii ABSTRAK xiv ABSTRACT xvi CHAPTER 1: INTRODUCTION