On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Pigments: the Third Step to Higher Quality And
Understanding Pigments: The Third Mark Harber October, 2000 Step to Higher Quality and Consistency Putting great color in your product is part of the pigments. However, they are less opaque and systems approach for resolving issues of sub- would have to be used at higher loading levels to standard properties and appearance. achieve similar whiteness and opacity. This article on pigments is the third in a four-part Titanium Dioxide is used in the majority of the series about the interrelationship of the material products made by the cast polymer industry. Tita- components used in marble and solid surface nium Dioxide-based colors include most whites, manufacturing. These AOC-authored articles re- pastels, earth tones and off-whites such as bone, spond to the challenge that the cast polymer in- ivory, beige or biscuit. As noted in Table 1, non- dustries aspire to higher standards of quality and white synthetic oxides are combined with Titani- consistency. Because resolving cast polymer is- um Dioxide to create pastels and earth tones for sues requires a systems approach, other articles cultured marble and solid surface applications. in this series address resins, gel coats and pro- cessing. All articles begin with background infor- Phthalocyanine pigments, or "Phthalos," impart mation on the main subject matter, followed by deep colors such as the automotive "Hunter ten related issues and guidelines. Green" of a sport utility vehicle or the high strength Blue used in ballpoint pens. Because A BACKGROUND ON COLORANTS they are so deep when used by themselves, In their natural state, cast polymer resins meet a Phthalo Blue and Phthalo Green are normally variety of performance requirements but are lack- blended with other pigments, many times Titani- ing in the color that draws the customer to the um Dioxide. -

Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=13374 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-25481-6 Toxicology; National Research Council 356 pages 6 x 9 PAPERBACK (2012) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. -

Nitrogen Dioxide
Common Name: NITROGEN DIOXIDE CAS Number: 10102-44-0 RTK Substance number: 1376 DOT Number: UN 1067 Date: May 1989 Revision: April 2000 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Nitrogen Dioxide can affect you when breathed in. * If you think you are experiencing any work-related health * Nitrogen Dioxide may cause mutations. Handle with problems, see a doctor trained to recognize occupational extreme caution. diseases. Take this Fact Sheet with you. * Contact can irritate and burn the skin and eyes with * Exposure to hazardous substances should be routinely possible eye damage. evaluated. This may include collecting personal and area * Breathing Nitrogen Dioxide can irritate the nose and air samples. You can obtain copies of sampling results throat. from your employer. You have a legal right to this * Breathing Nitrogen Dioxide can irritate the lungs causing information under OSHA 1910.1020. coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of WORKPLACE EXPOSURE LIMITS breath. OSHA: The legal airborne permissible exposure limit * High levels can interfere with the ability of the blood to (PEL) is 5 ppm, not to be exceeded at any time. carry Oxygen causing headache, fatigue, dizziness, and a blue color to the skin and lips (methemoglobinemia). NIOSH: The recommended airborne exposure limit is Higher levels can cause trouble breathing, collapse and 1 ppm, which should not be exceeded at any even death. time. * Repeated exposure to high levels may lead to permanent lung damage. ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift and IDENTIFICATION 5 ppm as a STEL (short term exposure limit). -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Emerging Technology
INDUSTRY NEWS EMERGING TECHNOLOGY Iron-based superconductors reinforce link to magnetism BRIEFS A new class of iron-oxyarsenide-based superconductors discovered earlier this year shares IDES Inc., a plastic similar unusual magnetic properties with previously known high-temperature superconductors materials information based on copper-oxide materials, report researchers at the National Institute of Standards and management company, Technology, Gaithersburg, Md. The work emphasizes a critical but as yet unexplained link be- and Firehole Technologies Inc., a tween magnetism and high-temperature superconductors. developer of innovative The importance of magnetism to high-temperature superconductors is remarkable because simulation technologies magnetism strongly interferes with conventional, low-temperature superconductors, but now for composite materials may prove to be an integral element of such materials. and structures, have The team used neutron beams to demonstrate that, like copper-oxide superconductors, the entered into a strategic new iron-oxyarsenide HTc materials discovered by Japanese researchers share an unusual partnership to develop a magnetic structure with magnetically active layers interspersed with layers of nonmagnetic searchable composite material. materials database. For more information: Qingzhen Huang, National Institute of Standards & Technology, 100 www.ides.com Bureau, Gaithersburg, MD 20899; tel: 301/ 975-6164; [email protected]; www.nist.gov. Intel Corp., Samsung Electronics, Copper nanowire arrays grown on different surfaces and Taiwan A simple process to grow upright copper nanowires on a variety of materials is under develop- Semiconductor ment by researchers at the University of Illinois in Urbana Champaign. The nanowire arrays Manufacturing could be suitable for field-emission displays, a new type of display technology that promises to pro- Company (TSMC) vide brighter, more vivid pictures than ex- have reached agreement on the need for industry- isting flat-panel displays. -
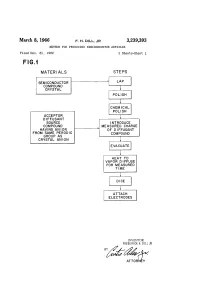
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD FOR PRODUCING SEMICONDUCTOR ARTICLES Filed Dec. 31, 1962 2. Sheets-Sheet FG. MATER ALS STEPS SEMCONDUCTOR COMPOUND CRYSTAL POLISH CHEMICAL POLISH ACCEPTOR DFFUSANT SOURCE INTRODUCE COMPOUND MEASURED CHARGE HAVING AN ON OF DFFUSANT FROM SAME PERODC COMPOUND GROUP AS CRYSTAL ANION EVACUATE HEAT TO VAPOR DIFFUSE FOR MEASURED TIME ATTACH ELECTRODES INVENTOR. FREDERICK H. DLL JR ATTORNEY March 8, 1966 F., H., DL, JR 3,239,393 METHOD FOR PRODUCING SEMCONDUCTOR ARTICLES Filed Dec. 31, 962 2. Sheets-Sheet 2 Šes is series NNNNN r N NNNNNNNN 3,239,393 United States Patent Office Patented Mar. 8, 1966 2. to produce precisely the desired results. For instance, 3,239,393 when metallic zinc is used as the diffusant material for METHOD FOR PRODUCING SEMCONDUCTOR a gallium arsenide substrate, it is very difficult to obtain ARTICLES the pure zinc metal with no zinc oxide film upon the metal. Frederick H. Dii, Jr., Patnam Waley, N.Y., assigor to 5 Furthermore, the metal is so tough that it is difficult to International Business Machines Corporation, New divide a pure metal sample into smaller pieces in order York, N.Y., a corporation of New York to obtain exactly the correct quantity for the diffusion Filed Dec. 31, 1962, Ser. No. 248,679 process. The zinc oxide on the surface of the metallic Zinc 7 Claims. (C. 48-189) diffusant material is very undesirable for a number of This invention relates to an improved diffusion process IO reasons. The oxygen is not wanted in the diffusion Vapor, for the production of Semiconductor devices, and more and the zinc oxide tends to form a protective coating particularly to an improved vapor diffusion process in over the zinc which inhibits the formation of the desired which the introduction of unwanted impurities is very zinc metal vapor which is required for the diffusion proc effectively and simply avoided, and which possesses other CSS. -
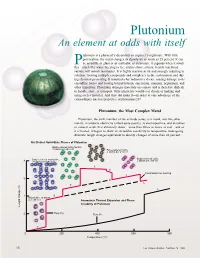
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

Organometallic Pnictogen Chemistry
Institut für Anorganische Chemie 2014 Fakultät für Chemie und Pharmazie | Sabine Reisinger aus Regensburg, geb. Scheuermayer am 15.07.1983 Studium: Chemie, Universität Regensburg Abschluss: Diplom Promotion: Prof. Dr. Manfred Scheer, Institut für Anorganische Chemie Sabine Reisinger Die vorliegende Arbeit enthält drei Kapitel zu unterschiedlichen Aspekten der metallorganischen Phosphor- und Arsen-Chemie. Zunächst werden Beiträge zur supramolekularen Chemie mit 5 Pn-Ligandkomplexen basierend auf [Cp*Fe(η -P5)] und 5 i [Cp*Fe(η - Pr3C3P2)] gezeigt, gefolgt von der Eisen-vermittelten Organometallic Pnictogen Aktivierung von P4, die zu einer selektiven C–P-Bindungsknüpfung führt, während das dritte Kapitel die Verwendung von Phosphor Chemistry – Three Aspects und Arsen als Donoratome in mehrkernigen Komplexen mit paramagnetischen Metallionen behandelt. Sabine Reisinger 2014 Alumniverein Chemie der Universität Regensburg E.V. [email protected] http://www.alumnichemie-uniregensburg.de Aspects Three – Chemistry Pnictogen Organometallic Fakultät für Chemie und Pharmazie ISBN 978-3-86845-118-4 Universität Regensburg Universitätsstraße 31 93053 Regensburg www.uni-regensburg.de 9 783868 451184 4 Sabine Reisinger Organometallic Pnictogen Chemistry – Three Aspects Organometallic Pnictogen Chemistry – Three Aspects Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Chemie und Pharmazie der Universität Regensburg vorgelegt von Sabine Reisinger, geb. Scheuermayer Regensburg 2014 Die Arbeit wurde von Prof. Dr. Manfred Scheer angeleitet. Das Promotionsgesuch wurde am 20.06.2014 eingereicht. Das Kolloquium fand am 11.07.2014 statt. Prüfungsausschuss: Vorsitzender: Prof. Dr. Helmut Motschmann 1. Gutachter: Prof. Dr. Manfred Scheer 2. Gutachter: Prof. Dr. Henri Brunner weiterer Prüfer: Prof. Dr. Bernhard Dick Dissertationsreihe der Fakultät für Chemie und Pharmazie der Universität Regensburg, Band 4 Herausgegeben vom Alumniverein Chemie der Universität Regensburg e.V. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

LOW LOSS ORIENTATION-PATTERNED GALLIUM ARSENIDE (Opgaas)
LOW LOSS ORIENTATION-PATTERNED GALLIUM ARSENIDE (OPGaAs) WAVEGUIDES FOR NONLINEAR INFRARED FREQUENCY CONVERSION Dissertation Submitted to The School of Engineering of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Doctor of Philosophy in Electrical and Computer Engineering By Izaak Vincent Kemp Dayton, Ohio December 2012 LOW LOSS ORIENTATION-PATTERNED GALLIUM ARSENIDE (OPGaAs) WAVEGUIDES FOR NONLINEAR INFRARED FREQUENCY CONVERSION Name: Kemp, Izaak Vincent APPROVED BY: Dr. Andrew Sarangan Dr. Peter Powers Advisory Committee Chairman Committee Member Professor Professor Electrical and Computer Engineering Department of Physics Dr. Partha Banerjee Dr. Guru Subramanyam Committee Member Committee Member Professor Department Chair Electrical and Computer Engineering Electrical and Computer Engineering Dr. Rita Peterson Dr. Kenneth Schepler Committee Member Committee Member Adjunct Professor Adjunct Professor Electro-Optics Electro-Optics John G. Weber, Ph.D. Tony E. Saliba, Ph.D. Associate Dean Dean, School of Engineering School of Engineering &Wilke Distinguished Professor ii © Copyright by Izaak Vincent Kemp All rights reserved 2012 iii Distribution Statement A: Approved for public release, distribution is unlimited. This dissertation contains information regarding currently ongoing U.S. Department of Defense (DoD) research that has been approved for public release. Distribution of this dissertation is unlimited pursuant to DoD Directive 5230.24 subsection A4. Requests for further information may be referred to the author, Izaak V. Kemp AFRL/RYMWA. iv ABSTRACT LOW LOSS ORIENTATION-PATTERNED GALLIUM ARSENIDE (OPGaAs) WAVEGUIDES FOR NONLINEAR INFRARED FREQUENCY CONVERSION Name: Kemp, Izaak Vincent University of Dayton Advisor: Dr. Andrew Sarangan The mid-IR frequency band (λ = 2-5 μm) contains several atmospheric transmission windows making it a region of interest for a variety of medical, scientific, commercial, and military applications. -

Creating New Superconducting & Semiconducting Nanomaterials And
Scholars' Mine Doctoral Dissertations Student Theses and Dissertations Summer 2013 Creating new superconducting & semiconducting nanomaterials and investingating the effect of reduced dimensionality on their properties Sukhada Mishra Follow this and additional works at: https://scholarsmine.mst.edu/doctoral_dissertations Part of the Chemistry Commons Department: Chemistry Recommended Citation Mishra, Sukhada, "Creating new superconducting & semiconducting nanomaterials and investingating the effect of reduced dimensionality on their properties" (2013). Doctoral Dissertations. 2060. https://scholarsmine.mst.edu/doctoral_dissertations/2060 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. CREATING NEW SUPERCONDUCTING & SEMICONDUCTING NANOMATERIALS AND INVESTINGATING THE EFFECT OF REDUCED DIMENSIONALITY ON THEIR PROPERTIES by SUKHADA MISHRA A DISSERTATION Presented to the Faculty of the Graduate School of the MISSOURI UNIVERSITY OF SCIENCE AND TECHNOLOGY In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in CHEMISTRY 2013 Approved by Dr. Manashi Nath, Advisor Dr. Philip Whitefield Dr. Nicholas Leventis Dr. Jeffrey Winiarz Dr. Matthew O’Keefe © 2013 Sukhada Mishra All rights reserved iii PUBLICATION DISSERTATION OPTION This dissertation consists of two published articles, two articles which have been submitted for publication and one manuscript in preparation. Paper I, found on pages 52—88, is published in ‗ACS Nano‘. Paper II included in pages 89—109, has been submitted for publication to ‗Advanced Materials‘. Paper III, found on pages 110—135, is published in ‗Nano Energy‘.