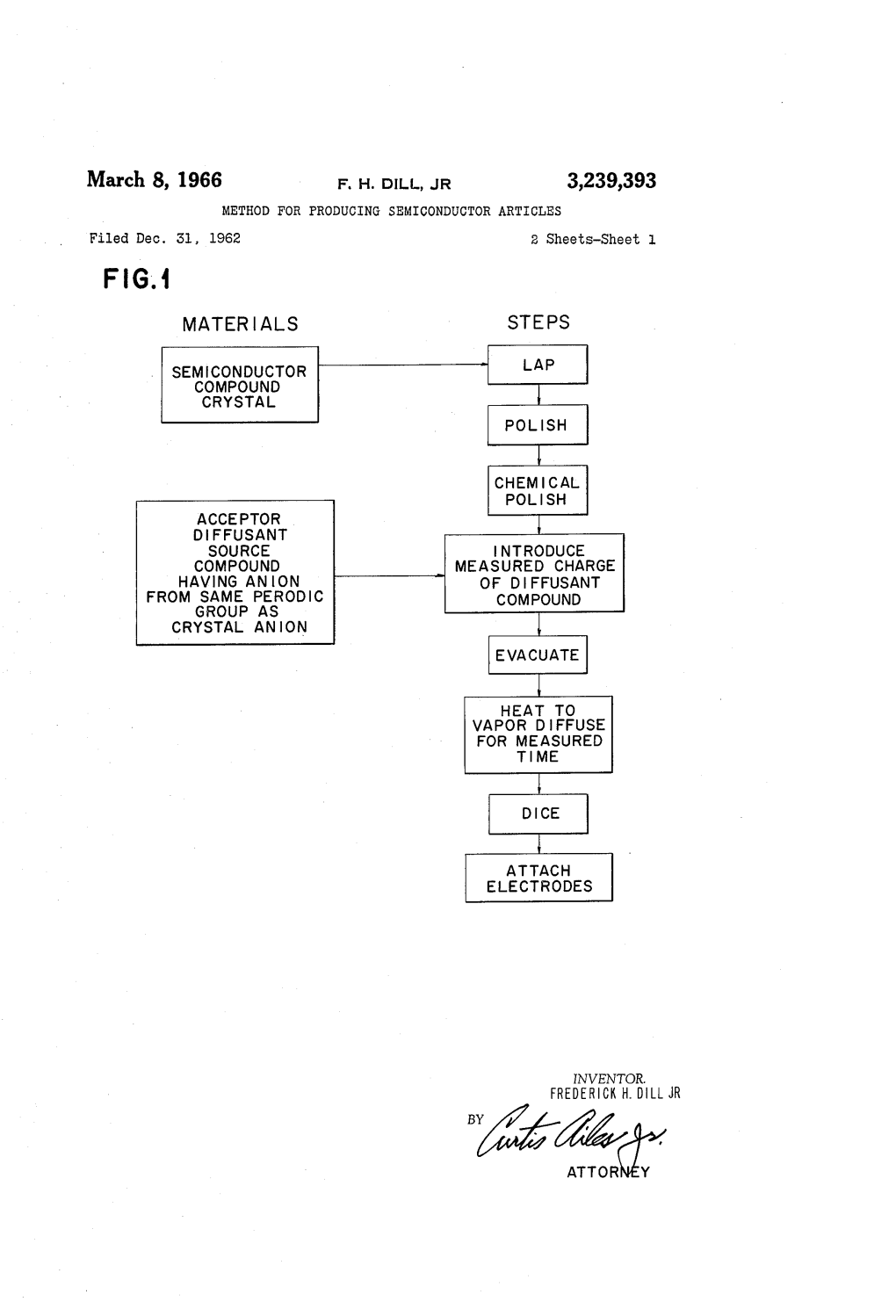
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re Et Al
US0083.33879B2 (12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re et al. (45) Date of Patent: Dec. 18, 2012 (54) ELECTRODEPOSITION OF DELECTRIC (56) References Cited COATINGS ON SEMCONDUCTIVE SUBSTRATES U.S. PATENT DOCUMENTS 3,455,806 A 7/1969 Spoor et al. (75) Inventors: Kelly L. Moore, Dunbar, PA (US); 3,663,389 A 5, 1972 Koral et al. Michael J. Pawlik, Glenshaw, PA (US); 3,749,657 A 7, 1973 Le Bras et al. Michael G. Sandala, Pittsburgh, PA 3,793,278 A 2f1974 De Bona (US); Craig A. Wilson, Allison Park, PA 3,928, 157 A 12/1975 Suematsu et al. 3,947.338 A 3, 1976 Jerabek et al. (US) 3,947,339 A 3, 1976 Jerabek et al. 3,962,165 A 6, 1976 Bosso et al. (73) Assignee: PPG Industries Ohio, Inc., Cleveland, 3,975,346 A 8, 1976 Bosso et al. OH (US) 3,984,299 A 10, 1976 Jerabek (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. EP OO12463 A1 6, 1980 (21) Appl. No.: 13/240,455 OTHER PUBLICATIONS (22) Filed: Sep. 22, 2011 Kohler, E. P. "An Apparatus for Determining Both the Quantity of Gas Evolved and the Amount of Reagent Consumed in Reactions (65) Prior Publication Data with Methyl Magnesium Iodide'. J. Am. Chem. Soc., 1927, 49 (12), US 2012/OOO6683 A1 Jan. 12, 2012 3181-3188, American Chemical Society, Washington, D.C. Related U.S. -

Safety Data Sheet
SAFETY DATA SHEET Revision Date 29-Jun-2018 Revision Number 1 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identification Product Description: Cadmium arsenide Cat No. : 22773 CAS-No 12006-15-4 Molecular Formula As2 Cd3 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Based on available data, the classification criteria are not met Health hazards Acute oral toxicity Category 3 (H301) Acute Inhalation Toxicity - Dusts and Mists Category 2 (H330) Environmental hazards ______________________________________________________________________________________________ ALFAA22773 Page 1 / 10 SAFETY DATA SHEET Cadmium arsenide Revision Date 29-Jun-2018 ______________________________________________________________________________________________ Acute aquatic toxicity Category 1 (H400) Chronic -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Thermomagnetic Effects in Cadmium Arsenide
Thermomagnetic effects in cadmium arsenide Citation for published version (APA): Blom, F. A. P. (1970). Thermomagnetic effects in cadmium arsenide. Technische Hogeschool Eindhoven. https://doi.org/10.6100/IR155506 DOI: 10.6100/IR155506 Document status and date: Published: 01/01/1970 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration. -

Topological Insulator State and Collapse of the Quantum Hall Effect in a Three-Dimensional Dirac Semimetal Heterojunction
PHYSICAL REVIEW X 10, 011050 (2020) Topological Insulator State and Collapse of the Quantum Hall Effect in a Three-Dimensional Dirac Semimetal Heterojunction David A. Kealhofer ,1 Luca Galletti ,2 Timo Schumann ,2 Alexey Suslov ,3 and Susanne Stemmer2,* 1Department of Physics, University of California, Santa Barbara, California 93106-9530, USA 2Materials Department, University of California, Santa Barbara, California 93106-5050, USA 3National High Magnetic Field Laboratory, Tallahassee, Florida 32310, USA (Received 10 July 2019; revised manuscript received 25 November 2019; accepted 23 December 2019; published 27 February 2020) Thin films promise new opportunities for the manipulation of surface states of topological semimetals with the potential to realize new states that cannot be obtained in bulk materials. Here, we report transport studies of gated Hall bar structures fabricated from approximately 50-nm-thick, (001)-oriented epitaxial films of cadmium arsenide, a prototype three-dimensional Dirac semimetal, in magnetic fields up to 45 T. The films exhibit a quantized Hall effect with pronounced odd-integer plateaus that is strikingly different from that of the more widely studied (112)-oriented films. We show that the unusual quantum Hall effect is a consequence of the inverted bulk band structure of cadmium arsenide that creates topological-insulator- like states at the bottom and top interfaces, each exhibiting a half-integer quantum Hall effect. A small potential offset between the two surfaces results in the crossing of the Landau levels and gives rise to the filling factor sequences observed in the experiments. Moreover, at large negative values of gate bias, the filling factor ν ¼ 1 is abruptly preempted by an insulating state that is accompanied by the collapse of the well-developed quantum Hall effect. -

Optical Constants of Amorphous Zinc Arsenide (Zn3as2) Via Spectroscopic Ellipsometry
Optical Constants of Amorphous Zinc Arsenide (Zn3As2) via Spectroscopic Ellipsometry James Colter Stewart A senior thesis submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Bachelor of Science John Colton, Advisor Department of Physics and Astronomy Brigham Young University April 2019 Copyright © 2019 James Colter Stewart All Rights Reserved ABSTRACT Optical Constants of Amorphous Zinc Arsenide (Zn3As2) via Spectroscopic Ellipsometry James Colter Stewart Department of Physics and Astronomy Bachelor of Science Zinc oxide (ZnO) is a promising wide band gap semiconductor with applications in ultraviolet optoelectronics. Through a novel sputtering process, our group seeks to create arsenic- doped p-type ZnO. In order to characterize these samples, we must understand the thin evaporated zinc arsenide (Zn3As2) layer in between the sputtered ZnO and our substrates. We characterize these samples by variable-angle spectroscopic ellipsometry (VASE), scanning electron microscopy (SEM), and X-ray diffraction (XRD). Enclosed is a paper our research group has submitted to Optics Express that reports the results of these characterizations; these results show that a five-parameter ellipsometric model is sufficient to find the optical constants for amorphous Zn3As2 samples. Further work needs to be done in order to properly characterize crystalline Zn3As2 samples via ellipsometry. Keywords: ellipsometry; zinc oxide; zinc arsenide; scanning electron microscopy (SEM); X-ray diffraction (XRD) ii Acknowledgments I would first like to thank my mentors Drs. John Colton and David Allred for their guidance and support of this project. Dr. Allred spent more than one Saturday with me on campus helping with the writing of the paper that comprises the bulk of this thesis. -

Field-Effect Transistors with the Three-Dimensional Dirac Semimetal Cadmium Arsenide
Field-effect transistors with the three- dimensional Dirac semimetal cadmium arsenide Cite as: Appl. Phys. Lett. 115, 062101 (2019); https://doi.org/10.1063/1.5103268 Submitted: 25 April 2019 . Accepted: 12 July 2019 . Published Online: 06 August 2019 Omor F. Shoron , Timo Schumann, Manik Goyal, David A. Kealhofer , and Susanne Stemmer Appl. Phys. Lett. 115, 062101 (2019); https://doi.org/10.1063/1.5103268 115, 062101 © 2019 Author(s). Applied Physics Letters ARTICLE scitation.org/journal/apl Field-effect transistors with the three-dimensional Dirac semimetal cadmium arsenide Cite as: Appl. Phys. Lett. 115, 062101 (2019); doi: 10.1063/1.5103268 Submitted: 25 April 2019 . Accepted: 12 July 2019 . Published Online: 6 August 2019 Omor F. Shoron,1 Timo Schumann,1 Manik Goyal,1 David A. Kealhofer,2 and Susanne Stemmer1,a) AFFILIATIONS 1Materials Department, University of California, Santa Barbara, California 93106-5050, USA 2Department of Physics, University of California, Santa Barbara, California 93106-9530, USA a)Email: [email protected] ABSTRACT Cadmium arsenide (Cd3As2) is a three-dimensional Dirac semimetal with many unique electronic properties that are of interest for future device applications. Here, we demonstrate field effect transistors using Cd3As2 as the channel material. We show that current densities exceed 5 A/mm and that very low contact resistances can be achieved even in unoptimized device structures. These properties make Cd3As2 of great interest for future high-speed electronics. We report on the current modulation characteristics of field effect transistors as a function of tem- perature. At low temperatures, the modulation exceeds 70%. We discuss material and device engineering approaches that can improve the device performance at room temperature. -

The University of Chicago Next Generation Synthetic
THE UNIVERSITY OF CHICAGO NEXT GENERATION SYNTHETIC ROUTES TO COLLOIDAL III-V QUANTUM DOTS A DISSERTATION SUBMITTED TO THE FACULTY OF THE DIVISION OF THE PHYSICAL SCIENCES IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF CHEMISTRY BY VISHWAS SRIVASTAVA CHICAGO, ILLINOIS AUGUST 2018 TABLE OF CONTENTS List of Figures ................................................................................................................... vii List of Tables ................................................................................................................... xiv Acknowledgments..............................................................................................................xv Abstract ........................................................................................................................... xvii CHAPTER 1: An Introduction to colloidal semiconductor nanocrystals ....................1 CHAPTER 2: Aminoarsines as arsenic precursors for the synthesis of metal arsenide nanocrystals ......................................................................................................................14 2.1 Introduction .................................................................................................................14 2.2 DIBAL-H mediated synthesis of InAs Nanocrystals ..................................................16 2.3 Effect of DIBAL-H concentration and temperature on NC size .................................18 2.4 Structural Characterization of InAs NCs ....................................................................21 -

Free Download
ALB Materials Inc 2360 Corporate Circle . Suite 400 Website: www.albmaterials.com Henderson, NV 89074-7739 E-mail: [email protected] Item No. Product Name CAS Number Formula Purity ALB‐semi‐AlSb Aluminum Antimonide [25152‐52‐7] AlSb 5N ALB‐semi‐Al2Se3 Aluminum Selenide [1302‐82‐5] Al2Se3 5N ALB‐semi‐Al2S3 Aluminum Sulfide [1302‐81‐4] Al2S3 5N ALB‐semi‐Al2Te3 Aluminum Telluride [12043‐29‐7] Al2Te3 5N ALB‐semi‐Sb Antimony [7440‐36‐0] Sb 5N, 6N ALB‐semi‐Sb2Se3 Antimony Selenide [1315‐05‐5] Sb2Se3 5N ALB‐semi‐Sb2S3 Antimony Sulfide [1345‐04‐6] Sb2S3 5N ALB‐semi‐Sb2Te3 Antimony Telluride [1327‐50‐0] Sb2Te3 4N, 5N ALB‐semi‐SbI3 Antimony(III) Iodide [7790‐44‐5] SbI3 4N ALB‐semi‐Sb2O3 Antimony(III) Oxide [1309‐64‐4] Sb2O3 5N ALB‐semi‐Sb2O5 Antimony(V) Oxide [1314‐60‐9] Sb2O5 5N ALB‐semi‐As2Se3 Arsenic Selenide [1303‐36‐2] As2Se3 5N ALB‐semi‐As2S3 Arsenic Sulfide [1303‐33‐9] As2S3 5N ALB‐semi‐Bi2O3 Bismuth Oxide [1304‐76‐3] Bi2O3 5N ALB‐semi‐Bi2Se3 Bismuth Selenide [12068‐69‐8] Bi2Se3 5N ALB‐semi‐Bi2S3 Bismuth Sulfide [1345‐07‐9] Bi2S3 5N ALB‐semi‐Bi2Te3 Bismuth Telluride [1304‐82‐1] Bi2Te3 4N, 5N ALB‐semi‐Cd Cadmium [7440‐43‐9]Inc Cd 5N, 6N, 7N ALB‐semi‐CdSb Cadmium Antimonide [12050‐27‐0] CdSb 5N ALB‐semi‐Cd3As2 Cadmium Arsenide [12006‐15‐4] Cd3As2 5N ALB‐semi‐CdSe Cadmium Selenide [1306‐24‐7] CdSe 4N, 5N ALB‐semi‐Cd2SnO4 Cadmium Stannate [12185‐56‐7] Cd2SnO4 5N ALB‐semi‐CdS Cadmium Sulfide [1306‐23‐6] CdS 4N, 5N ALB‐semi‐CdTe Cadmium Telluride [1306‐25‐8] CdTe 5N ALB‐semi‐Cu2S Copper Sulfide [22205‐45‐4] Cu2S 5N ALB‐semi‐GaSb Gallium Antimonide -

Semiconductor and Magnetic Material 3
Semiconductor and 11 Magnetic Material 2 Electronic Devices Circuits and Applications 1.1 INTRODUCTION Semiconductors are materials having electrical conductivities between those of good conductors and insulators. Semiconductors resistivity varies from 10–5 to 10+4 m. Similarly resistivity range values 10–8 to 10+6 m for conductors and from 107 to 108 m for insulators. Germanium (Ge) and Silicon (Si) are the most commonly used semiconductors and belong to Group-IV of the periodic table. They have resistivity of about 0.6 and 1.5 × 103 m respectively. Also there are certain compound semiconductors such as gallium arsenide (GaAs), indium phosphide (InP), cadmium sulphide (CdS), etc. They are formed by the combination of the elements of groups III and V. Small band gap is another important characteristic of semiconductors. Also the semiconductors have negative temperature coefficient of resistance because the number of carriers in a semiconductor will increase significantly with temperature, resulting in reduction of the resistance of the semiconductor. 1.2 SEMICONDUCTOR MATERIALS (GROUP-IV) Semiconductors are materials having electrical conductivities between those of good conductors and insulators. The elemental semiconductor such as germanium (Ge) and silicon (Si) belong to Group-IV of the periodic table and have resistivity of about 0.6 and 1.5 × 103 cm respectively. The energy band gaps of these elements of Group-IV at 0 K are given as below: C (diamond) → 5.51 eV Ge → 0.75 eV Si → 1.16 eV Sn (grey) → 0.08 eV Pd → ≈ eV We may say from above list that at room temperature (i.e., 0 K) diamond behaves as an insulator but Ge and Si are treated as semiconductors. -

Superconductivity Induced by Hydrogen Anion Substitution in 1111-Type Iron Arsenides
Superconductivity induced by hydrogen anion substitution in 1111-type iron arsenides Hideo Hosono,1,2,3* Satoru Matsuishi3 1Materials and Structures Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan 2Frontier Research Center, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan 3Materials Research Center for Element Strategy, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan *Corresponding author: Hideo Hosono Materials and Structures Laboratory, Tokyo Institute of Technology 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan TEL +81-45-924-5359 FAX +81-45-924-5339 E-mail: [email protected] Abstract Hydrogen is the simplest bipolar element and its valence state can be controlled from +1 to −1. We synthesized the 1111-type iron arsenides CaFeAsH and LnFeAsO1−xHx (Ln = lanthanide; 0 ≤ x ≤ 0.5) with the ZrCuSiAs type structure by a high-pressure synthesis method. The position and valence state of the substituted H were determined by neutron diffraction and density functional theory calculations. The close similarity in the structural and electrical properties of CaFeAsH and CaFeAsF indicated the formation of the hydride ion (H−), which is isovalent with the fluoride ion (F−), in the 1111-type iron arsenides. When some of the O2− ions in LnFeAsO are replaced by H−, superconductivity is induced by electron doping to the FeAs-layer to maintain charge neutrality. Since the substitution limit of hydrogen in LnFeAsO (x ≈ 0.5) is much higher than that of fluorine (x ≈ 0.2), the hydrogen substitution technique provides an effective pathway for high-density electron-doping, making it possible to draw the complete electronic phase diagram of LnFeAsO.