Superconductivity Induced by Hydrogen Anion Substitution in 1111-Type Iron Arsenides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CNSC Research Report 2016-17
The Science of Safety: CNSC Research Report 2016–17 © Canadian Nuclear Safety Commission (CNSC) 2018 Cat. No. CC171-24E-PDF ISSN 2369-4351 Extracts from this document may be reproduced for individual use without permission provided the source is fully acknowledged. However, reproduction in whole or in part for purposes of resale or redistribution requires prior written permission from the Canadian Nuclear Safety Commission. Également publié en français sous le titre : La science de la sûreté : Rapport de recherche de la CCSN 2016-2017 Document availability This document can be viewed on the CNSC website. To request a copy of the document in English or French, please contact: Canadian Nuclear Safety Commission 280 Slater Street P.O. Box 1046, Station B Ottawa, Ontario K1P 5S9 CANADA Tel.: 613-995-5894 or 1-800-668-5284 (in Canada only) Facsimile: 613-995-5086 Email: [email protected] Website: nuclearsafety.gc.ca Facebook: facebook.com/CanadianNuclearSafetyCommission YouTube: youtube.com/cnscccsn Twitter: @CNSC_CCSN Publishing History June 2018 Edition 1.0 Table of contents Message from the President .......................................................................................................................... 1 Introduction ................................................................................................................................................... 2 Ensuring the safety of nuclear power plants ................................................................................................. 6 Protecting -

Suppression Mechanisms of Alkali Metal Compounds
SUPPRESSION MECHANISMS OF ALKALI METAL COMPOUNDS Bradley A. Williams and James W. Fleming Chemistry Division, Code 61x5 US Naval Research Lnhoratory Washington, DC 20375-5342, USA INTRODUCTION Alkali metal compounds, particularly those of sodium and potassium, are widely used as fire suppressants. Of particular note is that small NuHCOi particles have been found to be 2-4 times more effective by mass than Halon 1301 in extinguishing both eountertlow flames [ I] and cup- burner flames [?]. Furthermore, studies in our laboratory have found that potassium bicarbonate is some 2.5 times more efficient by weight at suppression than sodium bicarhonatc. The primary limitation associated with the use of alkali metal compounds is dispersal. since all known compounds have very low volatility and must he delivered to the fire either as powders or in (usually aqueous) solution. Although powders based on alkali metals have been used for many years, their mode of effective- ness has not generally been agreed upon. Thermal effects [3],namely, the vaporization of the particles as well as radiative energy transfer out of the flame. and both homogeneous (gas phase) and heterogeneous (surface) chemistry have been postulated as mechanisms by which alkali metals suppress fires [4]. Complicating these issues is the fact that for powders, particle size and morphology have been found to affect the suppression properties significantly [I]. In addition to sodium and potassium, other alkali metals have been studied, albeit to a consider- ably lesser extent. The general finding is that the suppression effectiveness increases with atomic weight: potassium is more effective than sodium, which is in turn more effective than lithium [4]. -

Source of Atomic Hydrogen for Ion Trap Experiments: Review and Basic Properties
WDS'15 Proceedings of Contributed Papers — Physics, 155–161, 2015. ISBN 978-80-7378-311-2 © MATFYZPRESS Source of Atomic Hydrogen for Ion Trap Experiments: Review and Basic Properties A. Kovalenko, Š Roučka, S. Rednyk, T. D. Tran, D. Mulin, R. Plašil, J. Glosík Charles University in Prague, Faculty of Mathematics and Physics, Prague, Czech Republic. Abstract. The H-atom source was used to produce atomic hydrogen for study of ion-molecule reactions relevant to astrochemistry at low temperatures. H atoms were cooled and formed into an effusive beam, passing through a 22-pole ion trap. Here we present the basic operating principles of the H-atom source and give a review of this apparatus with some calculations of vacuum conditions and parameters of the produced H-atom beam. Introduction Hydrogen is the most abundant element in the Universe [Field et al., 1966]. It is the main component of stars, giant planets and interstellar clouds. Reactions with atomic or molecular hydrogen are important for understanding the processes which occur in the interstellar medium and during the formation of the new stars. There are three isotopes of hydrogen: protium, deuterium and tritium. The reactions of ions with H atoms have to be studied for better understanding of formation and destruction of more complex ions and molecules observed in interstellar medium. There are regions in space called H I and H II regions where hydrogen is mostly neutral in H I regions, rather than ionized or molecular in the H II regions. The atomic hydrogen plays fundamental role in many astrophysical contexts especially in formation H2 molecules [Habart et al., 2005; Sternberg et al., 2014]. -

Title Studies on Perovskite Oxyhydrides: Catalysis and Hydride
Studies on perovskite oxyhydrides: catalysis and hydride anion Title diffusion( Dissertation_全文 ) Author(s) Tang, Ya Citation 京都大学 Issue Date 2018-05-23 URL https://doi.org/10.14989/doctor.k21271 Right 許諾条件により本文は2018-12-30に公開 Type Thesis or Dissertation Textversion ETD Kyoto University Studies on perovskite oxyhydrides: catalysis and hydride anion diffusion Ya Tang 2018 Contents General Introduction 1 Chapter 1 Titanium-based hydrides as heterogeneous catalysts for ammonia synthesis 13 Chapter 2 Metal-dependent support effects of oxyhydride-supported Ru, Fe, Co catalysts for ammonia synthesis 33 Chapter 3 Hydride-enhanced CO2 methanation: water-stable BaTiO2.4H0.6 as a new support 60 Chapter 4 On hydride diffusion in transition metal perovskite oxyhydrides investigated via deuterium exchange 78 Chapter 5 General Conclusion 107 List of publications 109 Acknowledgment 111 General Introduction Background of This work Heterogeneous metal catalysts are vital to the chemical industry, especially in the conversion from fossil resources into fuels and a broad range of chemicals such as ammonia, methane, and methanol.1, 2 Typically, heterogeneous metal catalysts are consisting of several phases including metal particles and support materials. Normally, the metal particles are catalytically active phase in heterogeneous catalysts;3 for example, Fe or Ru in ammonia synthesis or Ni in catalytic hydrogenation reactions. In addition, however, support materials are extremely important and play a definitive role in determination of catalytic performance. Figure 1 illustrates a schematic model of the heterogeneous catalysis research.4 The importance of support effect is much in evidence in the heterogeneous catalysts; that is, the support materials help to stabilize the high dispersion of the metal particles,5, 6 or involves the catalytic reactions (i.e. -

Crystal Chemistry of Perovskite-Type Hydride Namgh3: Implications for Hydrogen Storage
Chem. Mater. 2008, 20, 2335–2342 2335 Crystal Chemistry of Perovskite-Type Hydride NaMgH3: Implications for Hydrogen Storage Hui Wu,*,†,‡ Wei Zhou,†,‡ Terrence J. Udovic,† John J. Rush,†,‡ and Taner Yildirim†,§ NIST Center for Neutron Research, National Institute of Standards and Technology, 100 Bureau DriVe, MS 6102, Gaithersburg, Maryland 20899-6102, Department of Materials Science and Engineering, UniVersity of Maryland, College Park, Maryland 20742-2115, and Department of Materials Science and Engineering, UniVersity of PennsylVania, 3231 Walnut Street, Philadelphia, PennsylVania 19104-6272 ReceiVed NoVember 26, 2007. ReVised Manuscript ReceiVed January 11, 2008 The crystal structure, lattice dynamics, and local metal-hydrogen bonding of the perovskite hydride NaMgH3 were investigated using combined neutron powder diffraction, neutron vibrational spectroscopy, and first-principles calculations. NaMgH3 crystallizes in the orthorhombic GdFeO3-type perovskite structure (Pnma) with a-b+a- octahedral tilting in the temperature range of 4 to 370 K. In contrast with previous structure studies, the refined Mg-H lengths and H-Mg-H angles indicate that the MgH6 octahedra maintain a near ideal configuration, which is corroborated by bond valence methods and our DFT calculations, and is consistent with perovskite oxides with similar tolerance factor values. The temperature dependences of the lattice distortion, octahedral tilting angle, and atomic displacement of H are also consistent with the recently observed high H mobility at elevated temperature. The stability and dynamics of NaMgH3 are discussed and rationalized in terms of the details of our observed perovskite structure. Further experiments reveal that its perovskite crystal structure and associated rapid hydrogen motion can be used to improve the slow hydrogenation kinetics of some strongly bound light-metal-hydride systems such as MgH2 and possibly to design new alloy hydrides with desirable hydrogen-storage properties. -

Highly Conductive Antiperovskites with Soft Anion Lattices 12 January 2021, by I
Highly conductive antiperovskites with soft anion lattices 12 January 2021, by I. Mindy Takamiya, Mari Toyama 'cation." They also have numerous intriguing properties, including superconductivity and, in contrast to most materials, contraction upon heating. Lithium- and sodium-rich antiperovskites, such as Li3OCl and Na3OCl, have been attracting much attention due to their high ionic conductivity and alkali metal concentration, making them promising candidates to replace liquid electrolytes used in lithium ion batteries. "But achieving a comparable lithium ion conductivity in solid materials has been challenging," explains iCeMS solid-state chemist Hiroshi Kageyama, who led the study. Soft anions, like sulfur ions (S2-), provide an ideal conduction path for sodium (Na+) and lithium (Li+) ions, Kageyama and his team synthesized a new family with the hydride ions (H-) helping to stabilize the of lithium- and sodium-rich antiperovskites that compound's structure. Credit: Mindy Takamiya/Kyoto begins to overcome this issue. Instead of 'hard' University iCeMS oxygen and halogen anions, their antiperovskites contain a hydrogen anion, called a hydride, and 'soft' chalcogen anions like sulfur. A new structural arrangement of atoms shows The scientists conducted a wide range of promise for developing safer batteries made with theoretical and experimental investigations on solid materials. Scientists at Kyoto University's these antiperovskites, and found that the soft anion Institute for Integrated Cell-Material Sciences lattice provides an ideal conduction path for lithium (iCeMS) designed a new type of 'antiperovskite' and sodium ions, which can be further enhanced by that could help efforts to replace the flammable chemical substitutions. organic electrolytes currently used in lithium ion batteries. -

Thermal and Structural Aspect of the Hydride Conducting Oxyhydride
Thermal and Structural Aspect of the Hydride Conducting Oxyhydride La2LiHO3 obtained via a Halide Flux Method Øystein S. Fjellvåg,† Jeff Armstrong,‡ Wojciech A. Slawinski‡ and Anja O. Sjåstad*,† †Centre for Materials Science and Nanotechnology, Department of Chemistry, University of Oslo, POBox 1033, N-0315 Oslo, Norway ‡ISIS Facility, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire OX11 0QX, U.K. 1 Abstract Oxyhydrides, in which oxide- and hydride- anions share the same anionic lattice, are relatively rare compounds. La2LiHO3 belongs to this family. We report the synthesis of La2LiHO3 by means of an alkali halide flux method, which allows the production of larger quantities of material relative to the usually adopted synthesis routes. Powder X-ray and neutron diffraction studies show that La2LiHO3 adopts the n = 1 Ruddlesden-Popper (RP) type structure with an orthorhombic distortion (space group Immm) due to hydride- and oxide- anion ordering. No sign of polymorphism is observed. La2LiHO3 is seen to decompose in an o oxygen atmosphere at 450 C into La2LiO3.5. We show that the high mobility of hydride anions close to the decomposition temperature is likely the main factor in inducing the oxidation. The crystal structure of La2LiO3.5 is also determined, and takes an RP n = 1 type structure with an orthorhombic distortion (Fmmm). This newly reported large scale synthesis approach, combined with the proven high thermal stability, are key factors for potential practical applications of this oxyhydride in real devices. 2 Introduction Hydrogen conducting materials have been widely studied for various technological applications. Examples include proton conductors 1, high temperature solid oxide fuel components and metals for hydrogen storage 2. -

PRESS RELEASE Source: Tokyo Institute of Technology for Immediate Release: December 22,2020 Turning the Heat Down: Catalyzing A
PRESS RELEASE Source: Tokyo Institute of Technology For immediate release: December 22,2020 Turning the Heat Down: Catalyzing Ammonia Formation at Lower Temperatures with Ruthenium (Tokyo, December 22) Scientists at Tokyo Institute of Technology (Tokyo Tech) report that the metal ruthenium, supported with lanthanide oxyhydrides, can efficiently catalyze the synthesis of ammonia at a much lower temperature than the traditional approach. In their new study, they highlight the advantages of the oxyhydride support and its potential in becoming a feasible catalyst for low-temperature ammonia synthesis in the future. Nitrogen is an essential nutrient for plant growth. While about 80% of earth is nitrogen, it is mostly contained in the atmosphere as gas, and hence, inaccessible to plants. To boost plant growth, especially in agricultural settings, therefore, chemical nitrogen fertilizers are needed. A crucial step in the production of these fertilizers is the synthesis of ammonia, which involves a reaction between hydrogen and nitrogen in the presence of a catalyst. Traditionally, ammonia production has been performed through the “Haber-Bosch” process, which, despite being effective, requires high temperature conditions (400–500°C), making the process expensive. Consequently, scientists have been trying to find a way to reduce the reaction temperatures of ammonia synthesis. Recently, scientists have reported ruthenium—a transition metal—as an efficient “catalyst” for ammonia synthesis, as it operates under milder conditions than traditional iron-based catalysts. However, there is a caveat: nitrogen molecules need to stick to the catalyst surface to undergo dissociation into atoms before reacting with hydrogen to form ammonia. For ruthenium, however, the low temperature often causes hydrogen molecules to stick to its surface instead—a process called hydrogen poisoning—which impedes the production of ammonia. -

Direct Synthesis of Chromium Perovskite Oxyhydride with a High Magnetictransition Temperature
Angewandte Chemie DOI: 10.1002/anie.201405453 Chromium Oxyhydride Direct Synthesis of Chromium Perovskite Oxyhydride with a High Magnetic-Transition Temperature** Cdric Tassel, Yoshihiro Goto, Yoshinori Kuno, James Hester, Mark Green, Yoji Kobayashi, and Hiroshi Kageyama* [7] Abstract: We report a novel oxyhydride SrCrO2H directly and cathode material. Their properties arise from the synthesized by a high-pressure high-temperature method. substitution of oxygen with other anions as well as the fine Powder neutron and synchrotron X-ray diffraction revealed tuning of the anionic and interacting d bands of the octahe- ÀÁthat this compound adopts the ideal cubic perovskite structure dral center B. Pm3m with O2À/HÀ disorder. Surprisingly, despite the non- Unlike the 2p based anions O2À,FÀ,S2À,N3À, the hydride À 2 bonding nature between Cr 3d t2g orbitals and the H 1s orbital, H anion is unique and only has a filled 1 s shell. Its it exhibits G-type spin ordering at TN 380 K, which is higher combination with a 2p anion as well as its interactions with the than that of RCrO3 (R = rare earth) and any chromium oxides. B element could be very interesting towards developing novel The enhanced TN in SrCrO2H with four Cr-O-Cr bonds in magnetic and transport properties. However, preparation of 3+ comparison with RCr O3 with six Cr-O-Cr bonds is reasonably explained by the tolerance factor. The present result offers an effective strategy to tune octahedral tilting in perovskites and to improve physical and chemical properties through mixed anion chemistry. Oxide perovskites ABO3 are the most encountered compounds in solid-state chemis- try. -

Characteristic Fast Hвˆ' Ion Conduction in Oxygen-Substituted Lanthanum
ARTICLE https://doi.org/10.1038/s41467-019-10492-7 OPEN Characteristic fast H− ion conduction in oxygen-substituted lanthanum hydride Keiga Fukui1, Soshi Iimura1, Tomofumi Tada 2, Satoru Fujitsu2, Masato Sasase2, Hiromu Tamatsukuri3, Takashi Honda 3,4, Kazutaka Ikeda3,4, Toshiya Otomo3,4 & Hideo Hosono1,2 Fast ionic conductors have considerable potential to enable technological development for energy storage and conversion. Hydride (H−) ions are a unique species because of their 1234567890():,; natural abundance, light mass, and large polarizability. Herein, we investigate characteristic H− conduction, i.e., fast ionic conduction controlled by a pre-exponential factor. Oxygen- −2 −1 doped LaH3 (LaH3−2xOx) has an optimum ionic conductivity of 2.6 × 10 Scm , which to the best of our knowledge is the highest H− conductivity reported to date at intermediate temperatures. With increasing oxygen content, the relatively high activation energy remains unchanged, whereas the pre-exponential factor decreases dramatically. This extraordinarily large pre-exponential factor is explained by introducing temperature-dependent enthalpy, derived from H− trapped by lanthanum ions bonded to oxygen ions. Consequently, light mass and large polarizability of H−, and the framework comprising densely packed H− in LaH3−2xOx are crucial factors that impose significant temperature dependence on the potential energy and implement characteristic fast H− conduction. 1 Laboratory for Materials and Structures, Tokyo Institute of Technology, Yokohama 226-8503, Japan. 2 Materials Research Center for Element Strategy, Tokyo Institute of Technology, Yokohama 226-8503, Japan. 3 Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK), Tsukuba 305-0801, Japan. 4 Department of Materials Structure Science, The Graduate University for Advanced Studies, Tsukuba 305-0801, Japan. -
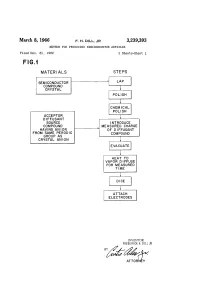
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD FOR PRODUCING SEMICONDUCTOR ARTICLES Filed Dec. 31, 1962 2. Sheets-Sheet FG. MATER ALS STEPS SEMCONDUCTOR COMPOUND CRYSTAL POLISH CHEMICAL POLISH ACCEPTOR DFFUSANT SOURCE INTRODUCE COMPOUND MEASURED CHARGE HAVING AN ON OF DFFUSANT FROM SAME PERODC COMPOUND GROUP AS CRYSTAL ANION EVACUATE HEAT TO VAPOR DIFFUSE FOR MEASURED TIME ATTACH ELECTRODES INVENTOR. FREDERICK H. DLL JR ATTORNEY March 8, 1966 F., H., DL, JR 3,239,393 METHOD FOR PRODUCING SEMCONDUCTOR ARTICLES Filed Dec. 31, 962 2. Sheets-Sheet 2 Šes is series NNNNN r N NNNNNNNN 3,239,393 United States Patent Office Patented Mar. 8, 1966 2. to produce precisely the desired results. For instance, 3,239,393 when metallic zinc is used as the diffusant material for METHOD FOR PRODUCING SEMCONDUCTOR a gallium arsenide substrate, it is very difficult to obtain ARTICLES the pure zinc metal with no zinc oxide film upon the metal. Frederick H. Dii, Jr., Patnam Waley, N.Y., assigor to 5 Furthermore, the metal is so tough that it is difficult to International Business Machines Corporation, New divide a pure metal sample into smaller pieces in order York, N.Y., a corporation of New York to obtain exactly the correct quantity for the diffusion Filed Dec. 31, 1962, Ser. No. 248,679 process. The zinc oxide on the surface of the metallic Zinc 7 Claims. (C. 48-189) diffusant material is very undesirable for a number of This invention relates to an improved diffusion process IO reasons. The oxygen is not wanted in the diffusion Vapor, for the production of Semiconductor devices, and more and the zinc oxide tends to form a protective coating particularly to an improved vapor diffusion process in over the zinc which inhibits the formation of the desired which the introduction of unwanted impurities is very zinc metal vapor which is required for the diffusion proc effectively and simply avoided, and which possesses other CSS. -

Cenixal0.5HZOY Nano-Oxyhydrides for H2 Production by Oxidative Dry Reforming of CH4 Without Carbon Formation
CeNiXAl0.5HZOY nano-oxyhydrides for H2 production by oxidative dry reforming of CH4 without carbon formation Yaqian Wei, Xiu Liu, Noura Haidar, Hervé Jobic, Sébastien Paul, Louise Jalowiecki-Duhamel To cite this version: Yaqian Wei, Xiu Liu, Noura Haidar, Hervé Jobic, Sébastien Paul, et al.. CeNiXAl0.5HZOY nano- oxyhydrides for H2 production by oxidative dry reforming of CH4 without carbon formation. Applied Catalysis A : General, Elsevier, 2020, 594, pp.117439. 10.1016/j.apcata.2020.117439. hal-03022420 HAL Id: hal-03022420 https://hal.archives-ouvertes.fr/hal-03022420 Submitted on 26 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. CeNiXAl0.5HZOY nano-oxyhydrides for H2 production by oxidative dry reforming of CH4 without carbon formation Yaqian Weia, Xiu Liua, Noura Haidar a, Hervé Jobicb, Sébastien Paula, and Louise Jalowiecki- Duhamela,* a Univ. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-59000 Lille, France. b Institut de Recherches sur la Catalyse et I’Environnement de Lyon (IRCELyon), 69626 Villeurbanne Cedex, France. Corresponding author. Tel.: +33 (0)3 20 33 77 35; fax: +33 (0)3 20 33 65 61.