Chemistry of the Main Group Elements: Chalcogens Through Noble Gases Sections 8.7-8.10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Periodic Trends in the Main Group Elements
Chemistry of The Main Group Elements 1. Hydrogen Hydrogen is the most abundant element in the universe, but it accounts for less than 1% (by mass) in the Earth’s crust. It is the third most abundant element in the living system. There are three naturally occurring isotopes of hydrogen: hydrogen (1H) - the most abundant isotope, deuterium (2H), and tritium 3 ( H) which is radioactive. Most of hydrogen occurs as H2O, hydrocarbon, and biological compounds. Hydrogen is a colorless gas with m.p. = -259oC (14 K) and b.p. = -253oC (20 K). Hydrogen is placed in Group 1A (1), together with alkali metals, because of its single electron in the valence shell and its common oxidation state of +1. However, it is physically and chemically different from any of the alkali metals. Hydrogen reacts with reactive metals (such as those of Group 1A and 2A) to for metal hydrides, where hydrogen is the anion with a “-1” charge. Because of this hydrogen may also be placed in Group 7A (17) together with the halogens. Like other nonmetals, hydrogen has a relatively high ionization energy (I.E. = 1311 kJ/mol), and its electronegativity is 2.1 (twice as high as those of alkali metals). Reactions of Hydrogen with Reactive Metals to form Salt like Hydrides Hydrogen reacts with reactive metals to form ionic (salt like) hydrides: 2Li(s) + H2(g) 2LiH(s); Ca(s) + H2(g) CaH2(s); The hydrides are very reactive and act as a strong base. It reacts violently with water to produce hydrogen gas: NaH(s) + H2O(l) NaOH(aq) + H2(g); It is also a strong reducing agent and is used to reduce TiCl4 to titanium metal: TiCl4(l) + 4LiH(s) Ti(s) + 4LiCl(s) + 2H2(g) Reactions of Hydrogen with Nonmetals Hydrogen reacts with nonmetals to form covalent compounds such as HF, HCl, HBr, HI, H2O, H2S, NH3, CH4, and other organic and biological compounds. -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Interhalogen Compounds
INTERHALOGEN COMPOUNDS Smt. EDNA RICHARD Asst. Professor Department of Chemistry INTERHALOGEN COMPOUND An interhalogen compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from any other group. Most interhalogen compounds known are binary (composed of only two distinct elements) The common interhalogen compounds include Chlorine monofluoride, bromine trifluoride, iodine pentafluoride, iodine heptafluoride, etc Interhalogen compounds into four types, depending on the number of atoms in the particle. They are as follows: XY XY3 XY5 XY7 X is the bigger (or) less electronegative halogen. Y represents the smaller (or) more electronegative halogen. Properties of Interhalogen Compounds •We can find Interhalogen compounds in vapour, solid or fluid state. • A lot of these compounds are unstable solids or fluids at 298K. A few other compounds are gases as well. As an example, chlorine monofluoride is a gas. On the other hand, bromine trifluoride and iodine trifluoride are solid and liquid respectively. •These compounds are covalent in nature. •These interhalogen compounds are diamagnetic in nature. This is because they have bond pairs and lone pairs. •Interhalogen compounds are very reactive. One exception to this is fluorine. This is because the A-X bond in interhalogens is much weaker than the X-X bond in halogens, except for the F-F bond. •We can use the VSEPR theory to explain the unique structure of these interhalogens. In chlorine trifluoride, the central atom is that of chlorine. It has seven electrons in its outermost valence shell. Three of these electrons form three bond pairs with three fluorine molecules leaving four electrons. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Chemistry of the Noble Gases*
CHEMISTRY OF THE NOBLE GASES* By Professor K. K. GREE~woon , :.\I.Sc., sc.D .. r".lU.C. University of N ewca.stle 1tpon Tyne The inert gases, or noble gases as they are elements were unsuccessful, and for over now more appropriately called, are a remark 60 years they epitomized chemical inertness. able group of elements. The lightest, helium, Indeed, their electron configuration, s2p6, was recognized in the gases of the sun before became known as 'the stable octet,' and this it was isolated on ea.rth as its name (i]A.tos) fotmed the basis of the fit·st electronic theory implies. The first inert gas was isolated in of valency in 1916. Despite this, many 1895 by Ramsay and Rayleigh; it was named people felt that it should be possible to induce argon (apy6s, inert) and occurs to the extent the inert gases to form compounds, and many of 0·93% in the earth's atmosphere. The of the early experiments directed to this end other gases were all isolated before the turn have recently been reviewed.l of the century and were named neon (v€ov, There were several reasons why chemists new), krypton (KpVn'TOV, hidden), xenon believed that the inert gases might form ~€vov, stmnger) and radon (radioactive chemical compounds under the correct con emanation). Though they occur much less ditions. For example, the ionization poten abundantly than argon they cannot strictly tial of xenon is actually lower than those of be called rare gases; this can be illustrated hydrogen, nitrogen, oxygen, fl uorine and by calculating the volumes occupied a.t s.t.p. -
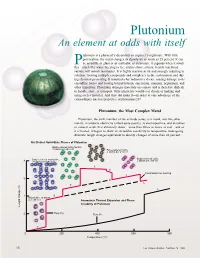
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

STRUCTURAL, ELECTRONIC PROPERTIES and the FEATURES of CHEMICAL BONDING in LAYERED 1111-OXYARSENIDES Larhaso and Lairaso: AB INITIO MODELING
The preprint. Submitted to: Journal of Structural Chemistry STRUCTURAL, ELECTRONIC PROPERTIES AND THE FEATURES OF CHEMICAL BONDING IN LAYERED 1111-OXYARSENIDES LaRhAsO AND LaIrAsO: AB INITIO MODELING V.V. Bannikov, I.R. Shein Institute of Solid State Chemistry, Ural Branch Of Russian Academy of Sciences, 620990, Ekaterinburg, Pervomayskaya St., 91, Russia E-mail: [email protected] The comparative study of structural, electronic properties, topology of the Fermi surface, and the features of chemical bonding in layered 1111-oxyarsenides LaRhAsO and LaIrAsO has been performed based on the results of ab initio modeling of their electronic structure. It was established that only weak sensitivity with respect both to electron and hole doping is expected for LaIrAsO being non-magnetic metal, however, the Rh-containing compound should be characterized with weak band magnetism, and the hole doping is expected to be able to move its ground state away from the boundary of magnetic instability. The mentioned feature allows to consider LaRhAsO oxyarsenide as a possible “electron analogue” of LaFeAsO compound being the initial phase for the layered FeAs-superconductors. Keywords: 1111-phases, oxyarsenides, doping, band structure, Fermi surface, chemical bonding INTRODUCTION The oxychalcogenides and oxypnictides with common chemical formula Me(a)Me(b)(Ch/Pn)O (where Me(a) is atom of Y, La, Bi or 4f-metal, usually taking the oxidation state +3 in compounds, Me(b) is atom of d-metal, Ch = S, Se, Te, Pn = P, As, Sb) form the vast family of so-called layered 1111-phases [1,2] with tetragonal ZrCuSiAs-like structure which can be regarded as the sequence of [Me(a)–O] and [Me(b)–(Ch/Pn)] blocks alternating along the tetragonal axis, where each atom is characterized with coordination number = 4 (Fig.1). -

Mean Amplitudes of Vibration of Iodine Trifluoride
Note 333 Mean Amplitudes of Vibration of Iodine Table 1. Calculated mean amplitudes of vibration (in A) of IF3. Trifluoride HK) MI-F(ax) «I-F(eq) u F(ax)...F(ax) MF(ax)...F(eq) E. J. Baran 0 0.0448 0.0401 0.057 0.057 Centro de Qufmica Inorgänica (CEQUINOR/CONICET, 100 0.0448 0.0401 0.057 0.058 UNLP), Facultad de Ciencias Exactas, 200 0.0460 0.0405 0.058 0.062 Universidad Nacional de La Plata, C. Correo 962, 298.16 0.0489 0.0420 0.061 0.068 1900-La Plata, Argentina 300 0.0489 0.0420 0.061 0.068 400 0.0528 0.0444 0.066 0.075 Reprint requests to Prof. E. J. B.; 500 0.0568 0.0474 0.071 0.082 E-mail: [email protected] 600 0.0609 0.0500 0.075 0.089 700 0.0648 0.0529 0.080 0.095 Z. Naturforsch. 56a, 333-334 (2001); 800 0.0686 0.0558 0.085 0.101 received January 12, 2001 900 0.0723 0.0586 0.089 0.107 1000 0.0759 0.0613 0.094 0.113 Mean amplitudes of vibration of IF3 have been calculated from vibrational spectroscopic data in the temperature range between 0 and 1000 K. Bond properties of the molecule are dis cussed on the basis of these results. Some comparison with relat Table 2. Comparison of the mean amplitudes of vibration (in A ed species are made. and at 298. 16 K) of the three isostructural XF3 species. -

1 Unit 4.4 Chemistry of Non-Transition Elements Few Facts of Halogens: A
Unit 4.4 Chemistry of Non-transition Elements Few facts of halogens: a) Ionisation energy of halogens is very high. This indicates that it has very little tendency to lose electrons. Due to gradual increase in size, it decreases down the group. b) The halogen molecules are held by weak Vander Waals forces, which increase down the group. This is responsible for the solid state of iodine. c) Chlorine has highest electron affinity in the group. Due to small size, fluorine has lower electron affinity than chlorine. d) All halogens are coloured as shown below: Halogens Fluorine Chlorine Bromine Iodine Colour Light Yellow Greenish Yellow Reddish Yellow Dark Violet The origin of colours of halogens is due to the absorption of visible light which excite the outermost electron to a higher energy level. Fluorine, being very small in size require very large excitation energy (obtained from the blue or violet part) of light, hence light yellow. Iodine being very large in size, the required lower excitation energy is obtained by absorption of yellow part of light. Interhalogen compounds: Halogen elements have different electro-negativity. Due to this they combine with each other to form covalent compounds (binary). “The binary compounds formed by halogens amongst themselves are known as Inter- halogen compounds”. These compounds have general formula; XYn, where n = 1, 3, 5 & 7. Ternary compounds of halogens are not known; as such a complex molecule might be unstable. Classification: Various types of inter-halogen compounds are tabulated below: Element Fluorine Chlorine Bromine Iodine Fluorine ------ ----- ----- ----- Chlorine ClF, ClF3, ClF5 --- --- --- Bromine BrF, BrF3, BrF5 BrCl --- --- Iodine IF, IF3, IF5, IF7 ICl, ICl3 IBr --- From the above table, the following points may be noted: • The inter-halogen compounds may be regarded as the halide of the more electronegative halogen. -

5.04 Principles of Inorganic Chemistry II Fall 2008
MIT OpenCourseWare http://ocw.mit.edu 5.04 Principles of Inorganic Chemistry II Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Chemistry 5.04 (F08) Practice Problem Set Practice vibrational and MO problems for the exam 1. Chatt prepared the dinitrogen complexes, trans-Mo(N2)2(PR3)4 in which the surprising result of two π-acid ligands coordinate trans to each other. Build the MO diagram for the complex. Draw the frontier d-orbital MOs and indicate the HOMO and LUMO orbitals. 2. Shown below are two electronically different metal carbides. Use MO arguments to address the following: a. Draw the HOMO for each complex. b. Explain the vacant site trans to the carbide atom in the square pyramidal, Ru complex. c. How many pi electrons are donated from the anilides into the metal in the moly complex (assume that for each sp2 hybirdized N-atom the tert-butly group point up, towards the apical carbide, and the aryl groups point down). Do the anilides and carbide compete for π symmetry orbitals on moly? d. Estimate the relative acidity of both carbide-carbon atoms. Which of these acids will protonate the carbide: MeC6H5, HCPh3, H3CCOOH, H(OEt2)B[3,5-C6H3(CF3)2] (answer yes or no for each acid/carbide combination)? 8 3. The synthesis of uranocene, [(η -C8H8)2U], is considered as the beginning of modern organoactinide chemistry. Organoactinides are distinguished by covalent interactions between the 5f orbitals of the 2– actinoids as well as the 6d orbitals. -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration.