Catalytic Oxidation of Sulfur Dioxide in Wastewater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Ruthenium Tetroxide and Perruthenate Chemistry. Recent Advances and Related Transformations Mediated by Other Transition Metal Oxo-Species
Molecules 2014, 19, 6534-6582; doi:10.3390/molecules19056534 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Ruthenium Tetroxide and Perruthenate Chemistry. Recent Advances and Related Transformations Mediated by Other Transition Metal Oxo-species Vincenzo Piccialli Dipartimento di Scienze Chimiche, Università degli Studi di Napoli ―Federico II‖, Via Cintia 4, 80126, Napoli, Italy; E-Mail: [email protected]; Tel.: +39-081-674111; Fax: +39-081-674393 Received: 24 February 2014; in revised form: 14 May 2014 / Accepted: 16 May 2014 / Published: 21 May 2014 Abstract: In the last years ruthenium tetroxide is increasingly being used in organic synthesis. Thanks to the fine tuning of the reaction conditions, including pH control of the medium and the use of a wider range of co-oxidants, this species has proven to be a reagent able to catalyse useful synthetic transformations which are either a valuable alternative to established methods or even, in some cases, the method of choice. Protocols for oxidation of hydrocarbons, oxidative cleavage of C–C double bonds, even stopping the process at the aldehyde stage, oxidative cleavage of terminal and internal alkynes, oxidation of alcohols to carboxylic acids, dihydroxylation of alkenes, oxidative degradation of phenyl and other heteroaromatic nuclei, oxidative cyclization of dienes, have now reached a good level of improvement and are more and more included into complex synthetic sequences. The perruthenate ion is a ruthenium (VII) oxo-species. Since its introduction in the mid-eighties, tetrapropylammonium perruthenate (TPAP) has reached a great popularity among organic chemists and it is mostly employed in catalytic amounts in conjunction with N-methylmorpholine N-oxide (NMO) for the mild oxidation of primary and secondary alcohols to carbonyl compounds. -

Selective Catalytic Oxidation of Ammonia to Nitrogen on Cuo-Ceo2 Bimetallic Oxide Catalysts
Hung, Aerosol and Air Quality Research, Vol. 6, No. 2, pp. 150-169, 2006 Selective Catalytic Oxidation of Ammonia to Nitrogen on CuO-CeO2 Bimetallic Oxide Catalysts Chang-Mao Hung* Department of Business Administration, Yung-Ta Institute of Technology & Commerce, 316 Chung-shan Road, Linlo, Pingtung 909, Taiwan, R.O.C. Abstract This study addresses the performance of the selective catalytic oxidation (SCO) of ammonia to N2 over a CuO-CeO2 bimetallic oxide catalyst in a tubular fixed-bed reactor (TFBR) at temperatures from 423 to 673 K in the presence of oxygen. CuO-CeO2 bimetallic oxide catalyst was prepared by co-precipitation with Cu(NO3)2 and Ce(NO3)3 at various molar concentrations. This study tested operational stability and investigated how the influent NH3 concentration (C0 = 500-1000 ppm) influences the capacity to remove NH3. The catalysts were characterized using XRD, FTIR, PSA, SEM and EDX. Ammonia was removed by oxidation in the absence of CuO- CeO2 bimetallic oxide catalyst, and the formation of copper (II) and cerium (IV) oxide active sites was confirmed. Additionally, the effects of the NH3 content of the carrier gas on the catalyst’s reaction rate (r) were observed. The results revealed that the extent of catalytic oxidation of ammonia in the presence of a CuO-CeO2 bimetallic oxide catalyst was a function of the molar ratio Cu:Ce in the bimetallic catalyst. The kinetics of catalyzed NH3 oxidation are described using the rate expression of the Eley-Rideal kinetic model. Also, experimental results indicate a reasonable mechanism for the catalytic oxidation of ammonia. -
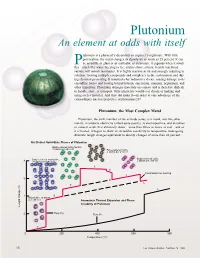
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 13 September 2018 doi:10.20944/preprints201809.0225.v1 Peer-reviewed version available at Catalysts 2018, 8, 483; doi:10.3390/catal8100483 Review General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis Sabine Valange 1,* and Jacques C. Védrine2,* 1 Institut de Chimie, des Milieux et Matériaux de Poitiers (IC2MP), UMR CNRS 7285, Université de Poitiers, ENSI Poitiers, B1, 1 rue Marcel Doré, TSA41105, F-86073, Poitiers Cedex 9, France 2 Sorbonne Université, Faculté des Sciences et Ingénierie, Laboratoire de Réactivité de Surface, UMR-CNRS 7197, 4 place Jussieu, F-75252 Paris, France * Correspondence: [email protected]; [email protected]; Tel.: +33-5-49454048 (S.V.); +33-1-44275560 (J.C.V.) Abstract: In this short review paper we have assembled the main characteristics of partial oxidation reactions (oxidative dehydrogenation and selective oxidation to olefins or oxygenates, as aldehydes and carboxylic acids and nitriles), as well as total oxidation, particularly for depollution, environmental issues and wastewater treatments. Both gas-solid and liquid-solid media have been considered with recent and representative examples within these fields. We have also discussed about their potential and prospective industrial applications. Particular attention has been brought to new raw materials stemming from biomass and to liquid-solid catalysts cases. This review paper also summarizes the progresses made in the use of unconventional activation methods for performing oxidation reactions, highlighting the synergy of these technologies with heterogeneous catalysis. Focus has been centered on usual catalysts activation methods but also on less usual ones, such as the use of ultrasounds, microwaves, grinding (mechanochemistry) and photo-activated processes, as well as their combined use. -

1 CHAPTER ONE Enantioselective Oxidation Chemistry 1.1 Oxidation
1 CHAPTER ONE Enantioselective Oxidation Chemistry 1.1 Oxidation in Biological Systems Oxidation is a fundamental process in chemistry and biology. In biological systems, a number of enzymes catalyze a diverse set of oxidative reactions through the use of molecular oxygen. Metalloenzymes that catalyze these aerobic oxidations are divided into two distinct types (Figure 1.1.1). Oxygenases involve the transfer of an oxygen atom from O2 to the substrate, often via a high-valent metal oxo species (1). The reduced metal intermediate (2) is then oxidized by dioxygen back to the active species, mediated by two protons and two electrons. Importantly, one of the oxygen atoms from O2 is incorporated into the oxidized substrate. Oxidases differ in that the metal oxidant (3) catalyzes the dehydrogenation of the substrate, releasing two protons. In this case, there is no oxygen atom transfer to the substrate—molecular oxygen is used simply as a proton and electron acceptor for the catalytic process. The atoms from O2 are released as either water or hydrogen peroxide. Figure 1.1.1 Oxygenase and oxidase metalloenzymes. Oxygenase enzymes: Oxidase enzymes: ox + Sub Sub(O) SubH2 Sub + 2 H O Mn+ M(n+2)+ Mn+ M(n+2)+ 1 2 3 4 O + 2 H+ H2O or 0.5 O2 or O2 H O 2 + 2 + 2 e– H2O2 + 2 H 2 1.2 Oxidation in Chemical Synthesis In chemical synthesis, there have been several developments regarding asymmetric oxidation catalysts that involve heteroatom transfer, i.e., catalysts that mimic the behavior of oxygenase enzymes. Some of the most notable examples are illustrated in Figure 1.2.1. -

Overview of Selective Oxidation of Ethylene to Ethylene Oxide by Ag Catalysts † ‡ † ‡ † Tiancheng Pu, Huijie Tian, Michael E
Review Cite This: ACS Catal. 2019, 9, 10727−10750 pubs.acs.org/acscatalysis Overview of Selective Oxidation of Ethylene to Ethylene Oxide by Ag Catalysts † ‡ † ‡ † Tiancheng Pu, Huijie Tian, Michael E. Ford, Srinivas Rangarajan,*, and Israel E. Wachs*, † ‡ Department of Chemical and Biomolecular Engineering and Operando Molecular Spectroscopy & Catalysis Laboratory, Lehigh University, Bethlehem, Pennsylvania 18015, United States ABSTRACT: Ethylene oxidation by Ag catalysts has been extensively investigated over the past few decades, but many key fundamental issues about this important catalytic system are still unresolved. This overview of the selective oxidation of ethylene to ethylene oxide by Ag catalysts critically examines the experimental and theoretical literature of this complex catalytic system: (i) the surface chemistry of silver catalysts (single crystal, α powder/foil, and supported Ag/ -Al2O3), (ii) the role of promoters, (iii) the reaction kinetics, (iv) the reaction mechanism, (v) density functional theory (DFT), and (vi) microkinetic modeling. Only in the past few years have the modern catalysis research tools of in situ/operando spectroscopy and DFT calculations been applied to begin establishing fundamental structure−activity/selectivity relationships. This overview of the ethylene oxidation reaction by Ag catalysts covers what is known and what issues still need to be determined to advance the rational design of this important catalytic system. KEYWORDS: ethylene epoxidation, silver, promoters, catalyst characterization, -

Catalytic Oxidation in Organic Synthesis
Catalytic Oxidation in Organic Synthesis Preface ............................................................................................................... V Volume Editor’s Preface ................................................................................ IX Abstracts ................................................. XI Table of Contents........................................................................................ XXV 1 Introduction K. Muniz ............................................................................................................. 1 2 General Concepts in Catalytic Oxidation .......................................................... 7 2.1 Photocatalytic Oxidation A. G. Griesbeck, S. Sillner, and M. Kleczka ............................................................ 7 2.2 Catalytic Oxidations with Hypervalent Iodine F. V. Singh and T. Wirth ....................................................................................... 29 2.3 Water as an Oxygen Source for Oxidation Reactions P. Garrido-Barros, I. Funes-Ardoiz, P. Farräs, C. Gimbert-Surinach, F. Maseras, and A. Llobet ....................................................................................................... 63 2.4 Dehydrogenation Y. Kayaki and T. Ikariya ........................................................................................ 81 2.5 Biomimetic Oxidation in Organic Synthesis L Vicens, M. Borrell, and M. Costas .................................................................. 113 3 Metal-Catalyzed Oxidation of -

Oxidation of Sulfur Dioxide to Sulfuric Acid Over Activated Carbon Catalyst Produced from Wood
392 Journal of the Japan Petroleum Institute, 46, (6), 392-395 (2003) [Research Note] Oxidation of Sulfur Dioxide to Sulfuric Acid over Activated Carbon Catalyst Produced from Wood Naonobu KATADA†1)*, Yusuke II†1), Munekazu NAKAMURA†2), and Miki NIWA†1) †1) Dept. of Materials Science, Faculty of Engineering, Tottori University, 4-101 Koyama-cho Minami, Tottori 680-8552, JAPAN †2) Faculty of Education and Regional Sciences, Tottori University, 4-101 Koyama-cho Minami, Tottori 680-8551, JAPAN (Received June 9, 2003) Sulfur dioxide can be removed from exhaust gases by oxidation to sulfuric acid over an activated carbon cata- lyst in the presence of water. A manufacturing method for activated carbon was developed based on the steam- ing of wood at relatively low temperature. The catalytic activity of the activated carbon produced from wood for the oxidation of sulfur dioxide was compared with those of commercially available activated carbons. The car- bon produced from wood showed high activity, but it was lower than that of a highly developed catalyst such as activated carbon fiber. However, the potential utilization of waste wood as an environmental catalyst was clear- ly demonstrated. Keywords Sulfur dioxide, Catalytic oxidation, Activated carbon catalyst, Waste wood, Sulfuric acid 1. Introduction was attempted5)~8). Recent studies of several catalysts such as activated carbon fiber9) and fluorocarbon-treat- Sulfur dioxide formed by the oxidation of sulfur ed carbon3) have found quite high performance. compounds in petroleum or coal burned as fuels causes Production of activated carbon from wood has acid rain. Several methods for the removal of sulfur recently become important for the utilization of waste compounds from the fuels or exhaust gases are utilized. -

STRUCTURAL, ELECTRONIC PROPERTIES and the FEATURES of CHEMICAL BONDING in LAYERED 1111-OXYARSENIDES Larhaso and Lairaso: AB INITIO MODELING
The preprint. Submitted to: Journal of Structural Chemistry STRUCTURAL, ELECTRONIC PROPERTIES AND THE FEATURES OF CHEMICAL BONDING IN LAYERED 1111-OXYARSENIDES LaRhAsO AND LaIrAsO: AB INITIO MODELING V.V. Bannikov, I.R. Shein Institute of Solid State Chemistry, Ural Branch Of Russian Academy of Sciences, 620990, Ekaterinburg, Pervomayskaya St., 91, Russia E-mail: [email protected] The comparative study of structural, electronic properties, topology of the Fermi surface, and the features of chemical bonding in layered 1111-oxyarsenides LaRhAsO and LaIrAsO has been performed based on the results of ab initio modeling of their electronic structure. It was established that only weak sensitivity with respect both to electron and hole doping is expected for LaIrAsO being non-magnetic metal, however, the Rh-containing compound should be characterized with weak band magnetism, and the hole doping is expected to be able to move its ground state away from the boundary of magnetic instability. The mentioned feature allows to consider LaRhAsO oxyarsenide as a possible “electron analogue” of LaFeAsO compound being the initial phase for the layered FeAs-superconductors. Keywords: 1111-phases, oxyarsenides, doping, band structure, Fermi surface, chemical bonding INTRODUCTION The oxychalcogenides and oxypnictides with common chemical formula Me(a)Me(b)(Ch/Pn)O (where Me(a) is atom of Y, La, Bi or 4f-metal, usually taking the oxidation state +3 in compounds, Me(b) is atom of d-metal, Ch = S, Se, Te, Pn = P, As, Sb) form the vast family of so-called layered 1111-phases [1,2] with tetragonal ZrCuSiAs-like structure which can be regarded as the sequence of [Me(a)–O] and [Me(b)–(Ch/Pn)] blocks alternating along the tetragonal axis, where each atom is characterized with coordination number = 4 (Fig.1).