Molybdenum Chalcogenides: Clusters, Chains, and Extended Solids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Is Selenium a Metal, Non-Metal Or Metalloid?
Is Selenium a metal, non-metal or metalloid? Abstract Is selenium(Se) a metal, non-metal, or a metalloid? There are various public opinions circulating around it. Since a long time from now, there are a lot of voices discussing this. Even until now, there is still no consensus about it. So, in this project, we are trying to find out whether selenium is a non-metal, metal or metalloids base on its physical and chemical properties which could be studied in the secondary school combining with the other information from the internet. Principles and hypothesis Studied from the secondary school chemistry, the general properties of metals include being good conductors of heat and electricity, having high melting and boiling points. Non-metals generally have a lower melting point and boiling point than metals and they being poor conductors of heat and electricity, etc. And the physical properties of metalloids are having extremely high melting/ boiling point, and having fair electrical conductivity. On the other hand, the oxides of metal are generally basic whereas the oxide of non-metals and metalloids are generally acidic. We will define selenium’s chemical category based on the above properties. Besides, we would like to introduce the concept of displacement reaction in studying the chemical properties of the chalcogens(e.g. sulphur(S) and selenium(Se)), especially selenium such rarely mentioned element. We can assume selenium is a metal if selenium could displace a metal oxide or a metal could displace selenium (IV) oxide. If selenium is a non-metal, its oxide could be displaced by sulphur which is supposed to be more reactive than selenium in the chalcogen group. -
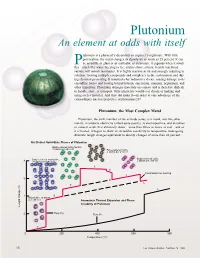
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH". -

Recent Developments in Chalcogen Chemistry
RECENT DEVELOPMENTS IN CHALCOGEN CHEMISTRY Tristram Chivers Department of Chemistry, University of Calgary, Calgary, Alberta, Canada WHERE IS CALGARY? Lecture 1: Background / Introduction Outline • Chalcogens (O, S, Se, Te, Po) • Elemental Forms: Allotropes • Uses • Trends in Atomic Properties • Spin-active Nuclei; NMR Spectra • Halides as Reagents • Cation Formation and Stabilisation • Anions: Structures • Solutions of Chalcogens in Ionic Liquids • Oxides and Imides: Multiple Bonding 3 Elemental Forms: Sulfur Allotropes Sulfur S6 S7 S8 S10 S12 S20 4 Elemental Forms: Selenium and Tellurium Allotropes Selenium • Grey form - thermodynamically stable: helical structure cf. plastic sulfur. R. Keller, et al., Phys. Rev. B. 1977, 4404. • Red form - cyclic Cyclo-Se8 (cyclo-Se7 and -Se6 also known). Tellurium • Silvery-white, metallic lustre; helical structure, cf. grey Se. • Cyclic allotropes only known entrapped in solid-state structures e.g. Ru(Ten)Cl3 (n = 6, 8, 9) M. Ruck, Chem. Eur. J. 2011, 17, 6382 5 Uses – Sulfur Sulfur : Occurs naturally in underground deposits. • Recovered by Frasch process (superheated water). • H2S in sour gas (> 70%): Recovered by Klaus process: Klaus Process: 2 H S + SO 3/8 S + 2 H O 2 2 8 2 • Primary industrial use (70 %): H2SO4 in phosphate fertilizers 6 Uses – Selenium and Tellurium Selenium and Tellurium : Recovered during the refining of copper sulfide ores Selenium: • Photoreceptive properties – used in photocopiers (As2Se3) • Imparts red color in glasses Tellurium: • As an alloy with Cu, Fe, Pb and to harden -

STRUCTURAL, ELECTRONIC PROPERTIES and the FEATURES of CHEMICAL BONDING in LAYERED 1111-OXYARSENIDES Larhaso and Lairaso: AB INITIO MODELING
The preprint. Submitted to: Journal of Structural Chemistry STRUCTURAL, ELECTRONIC PROPERTIES AND THE FEATURES OF CHEMICAL BONDING IN LAYERED 1111-OXYARSENIDES LaRhAsO AND LaIrAsO: AB INITIO MODELING V.V. Bannikov, I.R. Shein Institute of Solid State Chemistry, Ural Branch Of Russian Academy of Sciences, 620990, Ekaterinburg, Pervomayskaya St., 91, Russia E-mail: [email protected] The comparative study of structural, electronic properties, topology of the Fermi surface, and the features of chemical bonding in layered 1111-oxyarsenides LaRhAsO and LaIrAsO has been performed based on the results of ab initio modeling of their electronic structure. It was established that only weak sensitivity with respect both to electron and hole doping is expected for LaIrAsO being non-magnetic metal, however, the Rh-containing compound should be characterized with weak band magnetism, and the hole doping is expected to be able to move its ground state away from the boundary of magnetic instability. The mentioned feature allows to consider LaRhAsO oxyarsenide as a possible “electron analogue” of LaFeAsO compound being the initial phase for the layered FeAs-superconductors. Keywords: 1111-phases, oxyarsenides, doping, band structure, Fermi surface, chemical bonding INTRODUCTION The oxychalcogenides and oxypnictides with common chemical formula Me(a)Me(b)(Ch/Pn)O (where Me(a) is atom of Y, La, Bi or 4f-metal, usually taking the oxidation state +3 in compounds, Me(b) is atom of d-metal, Ch = S, Se, Te, Pn = P, As, Sb) form the vast family of so-called layered 1111-phases [1,2] with tetragonal ZrCuSiAs-like structure which can be regarded as the sequence of [Me(a)–O] and [Me(b)–(Ch/Pn)] blocks alternating along the tetragonal axis, where each atom is characterized with coordination number = 4 (Fig.1). -

Honors Chemistry ___/___/___
Name ____________________ Honors Chemistry ___/___/___ Chapter 3 Practice Test Part I: For each of the following, write the symbol of the element that best fits the description given. You may use an element more than once; you may even use it more than twice. (2 point each) 1. Li The alkali metal with the greatest ionization energy. 2. F The halogen with the greatest electronegativity. 3. Rn The noble gas with the greatest atomic radius. 4. Br The fourth period element with the highest electron affinity. 5. Pm The synthetic rare-earth element with the smallest atomic number. 6. Be The alkaline-earth element with the greatest second ionization energy. 7. Sb The fifth period metalloid with the largest atomic radius. 8. He The element on the periodic table with the smallest atomic radius. 9. Ar The third period non-metal with the highest ionization energy. 10. C The second period non-metal with the fewest valence electrons. 11. U The naturally occurring element with the greatest atomic mass. 12. Ne The second period element with the greatest ionization energy. 13. He The element with the greatest ionization energy. 14. Se The chalcogen non-metal with the greatest number of protons. 15. Al The third period metal with a 3+ oxidation number. 16. Fr The element with the lowest ionization energy. 17. Be The alkaline-earth element with the smallest atomic radius. 18. F The element with the greatest electronegativity. 19. At The sixth period element with the greatest electronegativity. 20. B The metalloid with the fewest valence electrons. -

Conceptual Progress for Explaining and Predicting Self-Organization on Anodized Aluminum Surfaces
nanomaterials Review Conceptual Progress for Explaining and Predicting Self-Organization on Anodized Aluminum Surfaces Mikhail Pashchanka Department of Chemistry, Eduard-Zintl-Institute, Technical University of Darmstadt, Alarich-Weiss-Straße 12, 64287 Darmstadt, Germany; [email protected] Abstract: Over the past few years, researchers have made numerous breakthroughs in the field of aluminum anodizing and faced the problem of the lack of adequate theoretical models for the interpretation of some new experimental findings. For instance, spontaneously formed anodic alumina nanofibers and petal-like patterns, flower-like structures observed under AC anodizing conditions, and hierarchical pores whose diameters range from several nanometers to sub-millimeters could be explained neither by the classical field-assisted dissolution theory nor by the plastic flow model. In addition, difficulties arose in explaining the basic indicators of porous film growth, such as the nonlinear current–voltage characteristics of electrochemical cells or the evolution of hexagonal pore patterns at the early stages of anodizing experiments. Such a conceptual crisis resulted in new multidisciplinary investigations and the development of novel theoretical models, whose evolution is discussed at length in this review work. The particular focus of this paper is on the recently developed electroconvection-based theories that allowed making truly remarkable advances in understanding the porous anodic alumina formation process in the last 15 years. Some explanation of the synergy between electrode reactions and transport processes leading to self-organization is provided. Finally, future prospects for the synthesis of novel anodic architectures are discussed. Citation: Pashchanka, M. Conceptual Progress for Explaining Keywords: porous anodic alumina (PAA); chaos and self-organization theory; electroconvection; and Predicting Self-Organization on colloidal gel model; anion exchange; DLVO theory; fluid mechanics; surface chemistry; surface Anodized Aluminum Surfaces. -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration. -

Elemental Sulfur Aerosol-Forming Mechanism
Elemental sulfur aerosol-forming mechanism Manoj Kumara and Joseph S. Franciscoa,1 aDepartment of Chemistry, University of Nebraska-Lincoln, Lincoln, NE 68588 Contributed by Joseph S. Francisco, December 21, 2016 (sent for review November 13, 2016; reviewed by James Lyons and Hua-Gen Yu) Elemental sulfur aerosols are ubiquitous in the atmospheres of Venus, reaction mechanism that may possibly convert the SOn + nH2S ancient Earth, and Mars. There is now an evolving body of evidence (n = 1, 2, 3) chemistries into the S8 aerosol in the gas phase suggesting that these aerosols have also played a role in the evolution (Scheme S1). It is the thermodynamics of these processes, and of early life on Earth. However, the exact details of their formation their catalysis by water and sulfuric acid, that we investigate here. mechanism remain an open question. The present theoretical calcula- This mechanism may not only help in better understanding the tions suggest a chemical mechanism that takes advantage of the in- role of sulfur cycle involving SOn,S8, and H2S as the potential S n = teraction between sulfur oxides, SOn ( 1, 2, 3) and hydrogen sulfide MIF carrier from the atmosphere to the ocean surface, but may 0 (nH2S), resulting in the efficient formation of a Sn+1 particle. Interest- also provide deeper insight into the formation mechanism of S + → + ingly, the SOn nH2S Sn+1 nH2O reactions occur via low-energy aerosols in various other environments. pathways under water or sulfuric acid catalysis. Once the S + particles n 1 We first explored the uncatalyzed gas-phase reactions of SOn with are formed, they may further nucleate to form larger polysulfur aero- nH2S using quantum-chemical calculations at the coupled cluster sols, thus providing a chemical framework for understanding the for- single and double substitution method with a perturbative treatment 0 mation mechanism of S aerosols in different environments. -

The Mechanism of Low-Temperature Oxidation of Carbon Monoxide by Oxygen Over the Pdcl2–Cucl2/Γ-Al2o3 Nanocatalyst
nanomaterials Article The Mechanism of Low-Temperature Oxidation of Carbon Monoxide by Oxygen over the PdCl2–CuCl2/γ-Al2O3 Nanocatalyst Lev Bruk 1, Denis Titov 1, Alexander Ustyugov 1, Yan Zubavichus 2 ID , Valeriya Chernikova 3, Olga Tkachenko 4, Leonid Kustov 4,*, Vadim Murzin 5, Irina Oshanina 1 and Oleg Temkin 1 1 Moscow Technological University, Institute of Fine Chemical Technology, Department of General Chemical Technology, Moscow 119571, Russia; [email protected] (L.B.); [email protected] (D.T.); [email protected] (A.U.); [email protected] (I.O.); [email protected] (O.T.) 2 National Research Centre “Kurchatov Institute”, Moscow 123182, Russia; [email protected] 3 Functional Materials Design, Discovery and Development Research Group (FMD3), Advanced Membranes and Porous Materials Center (AMPMC), Division of Physical Sciences and Engineering (PSE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia; [email protected] 4 N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 119991, Russia; [email protected] 5 Deutsches Elektronen-Synchrotron DESY, D-22607 Hamburg, Germany; [email protected] * Correspondence: [email protected]; Tel.: +7-499-137-2935 Received: 19 February 2018; Accepted: 30 March 2018; Published: 3 April 2018 Abstract: The state of palladium and copper on the surface of the PdCl2–CuCl2/γ-Al2O3 nanocatalyst for the low-temperature oxidation of CO by molecular oxygen was studied by various spectroscopic techniques. Using X-ray absorption spectroscopy (XAS), powder X-ray diffraction (XRD), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), freshly prepared samples of the catalyst were studied.