Recent Developments in Chalcogen Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Periodic Electronegativity Table
The Periodic Electronegativity Table Jan C. A. Boeyens Unit for Advanced Study, University of Pretoria, South Africa Reprint requests to J. C. A. Boeyens. E-mail: [email protected] Z. Naturforsch. 2008, 63b, 199 – 209; received October 16, 2007 The origins and development of the electronegativity concept as an empirical construct are briefly examined, emphasizing the confusion that exists over the appropriate units in which to express this quantity. It is shown how to relate the most reliable of the empirical scales to the theoretical definition of electronegativity in terms of the quantum potential and ionization radius of the atomic valence state. The theory reflects not only the periodicity of the empirical scales, but also accounts for the related thermochemical data and serves as a basis for the calculation of interatomic interaction within molecules. The intuitive theory that relates electronegativity to the average of ionization energy and electron affinity is elucidated for the first time and used to estimate the electron affinities of those elements for which no experimental measurement is possible. Key words: Valence State, Quantum Potential, Ionization Radius Introduction electronegative elements used to be distinguished tra- ditionally [1]. Electronegativity, apart from being the most useful This theoretical notion, in one form or the other, has theoretical concept that guides the practising chemist, survived into the present, where, as will be shown, it is also the most bothersome to quantify from first prin- provides a precise definition of electronegativity. Elec- ciples. In historical context the concept developed in a tronegativity scales that fail to reflect the periodicity of natural way from the early distinction between antag- the L-M curve will be considered inappropriate. -

Enhanced Heterogeneous Uptake of Sulfur Dioxide on Mineral Particles Through Modification of Iron Speciation During Simulated Cloud Processing
Atmos. Chem. Phys., 19, 12569–12585, 2019 https://doi.org/10.5194/acp-19-12569-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Enhanced heterogeneous uptake of sulfur dioxide on mineral particles through modification of iron speciation during simulated cloud processing Zhenzhen Wang1, Tao Wang1, Hongbo Fu1,2,3, Liwu Zhang1, Mingjin Tang4, Christian George5, Vicki H. Grassian6, and Jianmin Chen1 1Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Department of Environmental Science & Engineering, Institute of Atmospheric Sciences, Fudan University, Shanghai, 200433, China 2Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China 3Collaborative Innovation Center of Atmospheric Environment and Equipment Technology (CICAEET), Nanjing University of Information Science and Technology, Nanjing 210044, China 4State Key Laboratory of Organic Geochemistry and Guangdong Key Laboratory of Environmental Protection and Resources Utilization, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China 5University of Lyon, Université Claude Bernard Lyon 1, CNRS, IRCELYON, 69626, Villeurbanne, France 6Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093, USA Correspondence: Hongbo Fu ([email protected]) and Jianmin Chen ([email protected]) Received: 7 May 2019 – Discussion started: 5 June 2019 Revised: 7 August 2019 – Accepted: 5 September 2019 – Published: 9 October -

Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System
International Journal of Environmental Science and Development, Vol. 3, No. 4, August 2012 Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System Sabry M. Shaheen, Aly S. Derbalah, and Farahat S. Moghanm rich volcanic rocks (tuff) with fresh water in playa lakes or Abstract—In this study, the sorption behaviour of natural by seawater [5]. (clinoptilolite) zeolites with respect to cadmium (Cd), copper The structures of zeolites consist of three-dimensional (Cu), nickel (Ni), lead (Pb) and zinc (Zn) has been studied in frameworks of SiO and AlO tetrahedra. The aluminum ion order to consider its application to purity metal finishing 4 4 wastewaters. The batch method has been employed, using is small enough to occupy the position in the center of the competitive sorption system with metal concentrations in tetrahedron of four oxygen atoms, and the isomorphous 4+ 3+ solution ranging from 50 to 300 mg/l. The percentage sorption replacement of Si by Al produces a negative charge in and distribution coefficients (Kd) were determined for the the lattice. The net negative charge is balanced by the sorption system as a function of metal concentration. In exchangeable cation (sodium, potassium, or calcium). These addition lability of the sorbed metals was estimated by DTPA cations are exchangeable with certain cations in solutions extraction following their sorption. The results showed that Freundlich model described satisfactorily sorption of all such as lead, cadmium, zinc, and manganese [6]. The fact metals. Zeolite sorbed around 32, 75, 28, 99, and 59 % of the that zeolite exchangeable ions are relatively innocuous added Cd, Cu, Ni, Pb and Zn metal concentrations (sodium, calcium, and potassium ions) makes them respectively. -
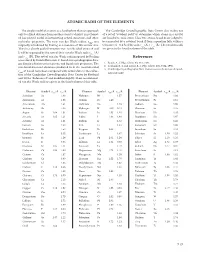
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -

Sulfur Safety Data Sheet SDS No: 6192 According to Federal Register / Vol
Sulfur Safety Data Sheet SDS No: 6192 According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations Revision Date: 10/23/2018 Date of Issue: 08/30/2012 Version: 1.0 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Mixture Product Name: Sulfur Synonyms: Brimstone, Sulfur 1.2. Intended Use of the Product Hydrogen sulfide may be present in trace quantities (by weight) in molten sulfur but may accumulate to toxic or flammable concentrations in enclosed spaces such as molten sulfur storage pits, tanks, or tanker/railcar headspaces. Hydrogen sulfide is not considered a hazard associated with solid sulfur. 1.3. Name, Address, and Telephone of the Responsible Party Customer Hess Tower 1501 McKinney Houston, TX 77010 T:(713) 496-4000 When calling the main operator ask for the EHS Safety Department. All Hess SDSs are also available via the Hess.com website. 1.4. Emergency Telephone Number Emergency Number : (800) 424-9300 CHEMTREC (24 hours) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture GHS-US Classification Flam. Sol. 2 H228 Skin Irrit. 2 H315 Aquatic Acute 2 H401 Comb. Dust Full text of hazard classes and H-statements : see Section 16. 2.2. Label Elements GHS-US Labeling Hazard Pictograms (GHS-US) : GHS02 GHS07 Signal Word (GHS-US) : Warning Hazard Statements (GHS-US) : May form combustible dust concentrations in air. H228 - Flammable solid. H315 - Causes skin irritation. H401 - Toxic to aquatic life. Precautionary Statements (GHS-US) : P210 - Keep away from heat, sparks, open flames, hot surfaces. - No smoking. P240 - Ground/Bond container and receiving equipment. -

On the Cycling of Sulfur and Mercury in the St. Louis River Watershed, Northeastern Minnesota
Page 1 of 91 Final Report On the Cycling of Sulfur and Mercury in the St. Louis River Watershed, Northeastern Minnesota An Environmental and Natural Trust Fund Final Report August 15, 2012 Michael Berndt and Travis Bavin Minnesota Department of Natural Resources 500 Lafayette Rd. St. Paul, MN 55455 Page 2 of 91 Final Report Contents Summary .......................................................................................................................................... 3 Introduction ..................................................................................................................................... 4 Methods ........................................................................................................................................... 5 Sampling Site Selection ................................................................................................................ 5 Chemical Analysis ......................................................................................................................... 6 34 18 Sulfur and Oxygen Isotopes in Dissolved Sulfate (δ SSO4 and δ OSO4)........................................ 7 Stream Gaging .............................................................................................................................. 7 Results .............................................................................................................................................. 7 Watershed Survey ....................................................................................................................... -

SO2 Removal by NH3 Gas Injection: Effects of Temperature and Moisture Content
Ind. Eng. Chem. Res. 1994,33, 1231-1236 1231 SO2 Removal by NH3 Gas Injection: Effects of Temperature and Moisture Content Hsunling Bai' Institute of Environmental Engineering, National Chiao- Tung University, Hsin-Chu, Taiwan, R.O.C. Pratim Biswas and Tim C. Keener Department of Civil and Environmental Engineering, University of Cincinnati, Cincinnati, Ohio 45221 -0071 The removal of SO2 by NH3 gas injection at various temperatures and moisture contents has been studied experimentally. The product compositions of the NH3-SO2-HzO vapor reactions were also reported. A thermodynamic analysis was carried out to predict the SO2 removal as well as the product compositions. Both the experimental results and thermodynamic analysis indicated that SO2 removal and the product compositions are sensitive to the reaction temperature. Moisture content, once in large excess of the stoichiometric requirement, does not have a strong effect on the product compositions but plays an important role in the SO2 removal. Introduction conditions. Stromberger (1984) used X-ray diffraction and identified the product particles to be (NH&S04 Sulfur dioxide removal from flue gases is a goal of many crystals. air pollution engineers. Significant efforts in the develop- ment of new flue gas desulfurization (FGD) technologies Although a number of studies on the removal of SO2 are being made. Major processes related to FGD tech- from flue gases by NH3 injection have been conducted, nologies include water scrubbing, metal ion solutions, there is disagreement on the operating condition (such as catalytic oxidation, dry or semidry adsorption, wet lime temperature) that a high removal efficiency can be or limestone scrubbing, double alkali process, and ammonia obtained. -

Sodium Chlorite Sulfur Destruction
® Basic Chemicals Sodium Chlorite Sulfur Destruction Application Description: Advantages of Sodium Chlorite/Chlorine Reduced sulfur compounds are a broad Dioxide: class of oxy-sulfur compounds, such as = = sulfite (SO3 ) and thiosulfate (S2O3 ), that Chlorine dioxide reacts the most rapidly have an oxidant demand. These and does not form chlorinated organic compounds are found in the waste streams by-products. of the petroleum, steel, paper and most While chlorine is the least expensive chemical industries. Their high oxidant chemical, it cannot be used when demand can cause eutrophication of natural organic compounds are present due to waters and excessive chlorine demand in the formation of chlorinated organic by- wastewaters treated by POTWs (Publicly products. Owned Treatment Works). When chlorine can't be used, hydrogen peroxide has the lowest chemical costs. Chlorine dioxide effectively oxidizes these species to sulfate ions over a broad pH range (5-9). Below a pH of 4, sodium Affected Industries: chlorite may be used without the generation Chemicals, Food, Iron & Steel, Mining, Oil of chlorine dioxide. Since these compounds Refining, Plastics & Rubber, Pulp & Paper, are usually found in mixtures of various Textiles ratios the required chlorine dioxide dosage must be determined for each application. Further Information More detailed information on sodium Alternatives: chlorite applications is available upon Hydrogen peroxide solution is added by request through the OxyChem Technical a chemical dosing pump. Services Department. Call or write to: Chlorine gas is added by a vacuum eductor system, while sodium OxyChem hypochlorite solution is added by a Technical Service Department chemical dosing pump. PO Box 12283 Wichita, Kansas 67277-2283 800-733-1165 Ext. -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Most Atoms Form Chemical Bonds to Obtain a Lower Potential Energy
Integrated Chemistry <Kovscek> 6-1-2 Explain why most atoms form chemical bonds. Most atoms form chemical bonds to obtain a lower potential energy. Many share or transfer electrons to get a noble gas configuration. 6-1-3 Describe ionic and covalent bonding. Ions form to lower the reactivity of an atom. Ionic bonding is when the attraction between many cations and anions hold atoms together. Covalent bonding results from the sharing of electron pairs between two atoms. That is, when atoms share electrons, the mutual attraction between the electrons (electron clouds) and protons (the nucleus) hold the atoms together. 6-1-4 Explain why most chemical bonding is neither purely ionic nor purely covalent. In order for a bond to be totally ionic one atom would have to have an electronegativity of zero. (there aren’t any). To have a purely covalent bond the electronegativity must be equal. (Only the electronegativity of atoms of the same element are identical, diatomic elements are purely covalent) 6-1-5 Classify bonding type according to electronegativity differences. Differences in electronegativities reflect the character of bonding between elements. The electronegativity of the less- electronegative element is subtracted from that of the more- electronegative element. The greater the electronegativity difference, the more ionic is the bonding. Covalent Bonding happens between atoms with an electronegativity difference of 1.7 or less. That is the bond has an ionic character of 50% or less. Electronegativity % Ionic Character Covalent Differences Bond Type 0 to 0.3 0% to 5% Non - Polar Over 0.3 up to 1.7 5% to 50% Polar Over 1.7 Greater than 50 % Ionic Ionic Bonding occurs between atoms with an electronegativity difference of greater than 1.7. -

ELECTRONEGATIVITY D Qkq F=
ELECTRONEGATIVITY The electronegativity of an atom is the attracting power that the nucleus has for it’s own outer electrons and those of it’s neighbours i.e. how badly it wants electrons. An atom’s electronegativity is determined by Coulomb’s Law, which states, “ the size of the force is proportional to the size of the charges and inversely proportional to the square of the distance between them”. In symbols it is represented as: kq q F = 1 2 d 2 where: F = force (N) k = constant (dependent on the medium through which the force is acting) e.g. air q1 = charge on an electron (C) q2 = core charge (C) = no. of protons an outer electron sees = no. of protons – no. of inner shell electrons = main Group Number d = distance of electron from the nucleus (m) Examples of how to calculate the core charge: Sodium – Atomic number 11 Electron configuration 2.8.1 Core charge = 11 (no. of p+s) – 10 (no. of inner e-s) +1 (Group i) Chlorine – Atomic number 17 Electron configuration 2.8.7 Core charge = 17 (no. of p+s) – 10 (no. of inner e-s) +7 (Group vii) J:\Sciclunm\Resources\Year 11 Chemistry\Semester1\Notes\Atoms & The Periodic Table\Electronegativity.doc Neon holds onto its own electrons with a core charge of +8, but it can’t hold any more electrons in that shell. If it was to form bonds, the electron must go into the next shell where the core charge is zero. Group viii elements do not form any compounds under normal conditions and are therefore given no electronegativity values.