The Oxidation of Rhenium and Identification of Rhenium Oxides During Catalytic Partial Oxidation of Ethylene: an In-Situ XPS Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Pigments: the Third Step to Higher Quality And
Understanding Pigments: The Third Mark Harber October, 2000 Step to Higher Quality and Consistency Putting great color in your product is part of the pigments. However, they are less opaque and systems approach for resolving issues of sub- would have to be used at higher loading levels to standard properties and appearance. achieve similar whiteness and opacity. This article on pigments is the third in a four-part Titanium Dioxide is used in the majority of the series about the interrelationship of the material products made by the cast polymer industry. Tita- components used in marble and solid surface nium Dioxide-based colors include most whites, manufacturing. These AOC-authored articles re- pastels, earth tones and off-whites such as bone, spond to the challenge that the cast polymer in- ivory, beige or biscuit. As noted in Table 1, non- dustries aspire to higher standards of quality and white synthetic oxides are combined with Titani- consistency. Because resolving cast polymer is- um Dioxide to create pastels and earth tones for sues requires a systems approach, other articles cultured marble and solid surface applications. in this series address resins, gel coats and pro- cessing. All articles begin with background infor- Phthalocyanine pigments, or "Phthalos," impart mation on the main subject matter, followed by deep colors such as the automotive "Hunter ten related issues and guidelines. Green" of a sport utility vehicle or the high strength Blue used in ballpoint pens. Because A BACKGROUND ON COLORANTS they are so deep when used by themselves, In their natural state, cast polymer resins meet a Phthalo Blue and Phthalo Green are normally variety of performance requirements but are lack- blended with other pigments, many times Titani- ing in the color that draws the customer to the um Dioxide. -

Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=13374 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-25481-6 Toxicology; National Research Council 356 pages 6 x 9 PAPERBACK (2012) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. -

Nitrogen Dioxide
Common Name: NITROGEN DIOXIDE CAS Number: 10102-44-0 RTK Substance number: 1376 DOT Number: UN 1067 Date: May 1989 Revision: April 2000 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Nitrogen Dioxide can affect you when breathed in. * If you think you are experiencing any work-related health * Nitrogen Dioxide may cause mutations. Handle with problems, see a doctor trained to recognize occupational extreme caution. diseases. Take this Fact Sheet with you. * Contact can irritate and burn the skin and eyes with * Exposure to hazardous substances should be routinely possible eye damage. evaluated. This may include collecting personal and area * Breathing Nitrogen Dioxide can irritate the nose and air samples. You can obtain copies of sampling results throat. from your employer. You have a legal right to this * Breathing Nitrogen Dioxide can irritate the lungs causing information under OSHA 1910.1020. coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of WORKPLACE EXPOSURE LIMITS breath. OSHA: The legal airborne permissible exposure limit * High levels can interfere with the ability of the blood to (PEL) is 5 ppm, not to be exceeded at any time. carry Oxygen causing headache, fatigue, dizziness, and a blue color to the skin and lips (methemoglobinemia). NIOSH: The recommended airborne exposure limit is Higher levels can cause trouble breathing, collapse and 1 ppm, which should not be exceeded at any even death. time. * Repeated exposure to high levels may lead to permanent lung damage. ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift and IDENTIFICATION 5 ppm as a STEL (short term exposure limit). -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Emerging Technology
INDUSTRY NEWS EMERGING TECHNOLOGY Iron-based superconductors reinforce link to magnetism BRIEFS A new class of iron-oxyarsenide-based superconductors discovered earlier this year shares IDES Inc., a plastic similar unusual magnetic properties with previously known high-temperature superconductors materials information based on copper-oxide materials, report researchers at the National Institute of Standards and management company, Technology, Gaithersburg, Md. The work emphasizes a critical but as yet unexplained link be- and Firehole Technologies Inc., a tween magnetism and high-temperature superconductors. developer of innovative The importance of magnetism to high-temperature superconductors is remarkable because simulation technologies magnetism strongly interferes with conventional, low-temperature superconductors, but now for composite materials may prove to be an integral element of such materials. and structures, have The team used neutron beams to demonstrate that, like copper-oxide superconductors, the entered into a strategic new iron-oxyarsenide HTc materials discovered by Japanese researchers share an unusual partnership to develop a magnetic structure with magnetically active layers interspersed with layers of nonmagnetic searchable composite material. materials database. For more information: Qingzhen Huang, National Institute of Standards & Technology, 100 www.ides.com Bureau, Gaithersburg, MD 20899; tel: 301/ 975-6164; [email protected]; www.nist.gov. Intel Corp., Samsung Electronics, Copper nanowire arrays grown on different surfaces and Taiwan A simple process to grow upright copper nanowires on a variety of materials is under develop- Semiconductor ment by researchers at the University of Illinois in Urbana Champaign. The nanowire arrays Manufacturing could be suitable for field-emission displays, a new type of display technology that promises to pro- Company (TSMC) vide brighter, more vivid pictures than ex- have reached agreement on the need for industry- isting flat-panel displays. -

The Oxidation of Carbon Monoxide Using a Tin Oxide
THE OXIDATION OF CARBON MONOXIDE USING A TIN OXIDE CATALYST Christopher F. Sampson and Nicholas J. Gudde United Kingdom Atomic Energy Authority A.E.R.E. Harwell, Didcot, Oxon. United Kingdom SUMMARY This paper outlines some of the steps involved in the development by the United Kingdom Atomic Energy Authority (UKAEA) of a catalytic device for the recombination of carbon monoxide and oxygen in a C02 laser system. It contrasts the differences between CO oxidation for air purification and for laser environmental control, but indicates that there are similarities between the physical specifications. The principal features of catalytic devices are outlined and some experimental work described. This includes measurements concerning the structure and mechanical properties of the artifact, the preparation of the catalyst coating and its interaction with the gaseous environment. The paper concludes with some speculation about the method by which the reaction actually occurs. INTRODUCTION During the late 1970's, the United Kingdom Atomic Energy Authority at Harwell became involved in sol-gel technology as a result of oxide fuel development. The sol-gel method was found to be suitable for the preparation of catalytic materials which eventually led to their use in car exhaust catalytic converters and for air purification catalysts. Such catalysts were usually in the form of coatings applied to supporting artifacts such as monoliths, formed from either cordierite or from a corrosion resistant metal such as Fecralloy steel (R). The UKAEA has experience in the use of sol-gel catalysts for CO removal from air and so has a technological link to a system intended to recombine O2 and CO formed in sealed C02 lasers. -

Zinc Oxide Sulfide Scavenger Contains a High-Quality Zinc Oxide
ZINC OXIDE ZINC OXIDE sulfide scavenger contains a high-quality ZINC OXIDE. The very fine particle-size of ZINC OXIDE scavenger results in a maximum amount of surface area for fast, efficient sulfide scavenging. It reacts with sulfides (see APPLICATIONS below) to form ZnS. This precipitate is an insoluble, inert, fine solid that remains harmlessly in the mud system or is removed by the solids-control equipment. Typical Physical Properties Physical appearance ������������������������������������������������������������������������������������������������������������������������������������������������������������������������������ White to off-white powder Specific gravity .......................................................................................................................................................................................................................... 5.4 – 5.6 Bulk density ........................................................................................................................................................................................................ 164 lb/ft3 (2627 kg/m3) Applications Under operating conditions, ZINC OXIDE scavenger reacts with sulfides to form ZnS, as shown in these equations: Zn2+ + HS- + OH- → ZnS ↓ + 2+ 2- H2O Zn + S → ZnS ↓ INC XIDE Z O scavenger is effective at the pH levels found in drilling fluids. It is recommended that a pH above 11 be maintained whenever H2S is - 2- expected. This high alkalinity converts the dangerous H2S gas to less toxic bisulfide -

Sulfur Dioxide Emissions
Report on the Environment https://www.epa.gov/roe/ Sulfur Dioxide Emissions Sulfur dioxide (SO2) belongs to the family of sulfur oxide (SOx) gases. These gases are formed when fuel containing sulfur (mainly coal and oil) is burned (e.g., for electricity generation) and during metal smelting and other industrial processes. High concentrations of SO2 are associated with multiple health and environmental effects. The highest concentrations of SO2 have been recorded in the vicinity of large industrial facilities. SO2 emissions are an important environmental issue because they are a major precursor to ambient PM2.5 concentrations. Short-term exposure to airborne SO2 has been associated with various adverse health effects (ATSDR, 1998; U.S. EPA, 2017). Multiple human clinical studies, epidemiological studies, and toxicological studies support a causal relationship between short-term exposure to airborne SO2 and respiratory morbidity. The observed health effects have included respiratory symptoms, airway inflammation, and increased emergency department visits and hospitalizations for all respiratory causes. These studies further suggest that asthmatics, children, older adults, and people who spend a lot of time outdoors at increased exertion levels are potentially susceptible or vulnerable to these health effects (U.S. EPA, 2017). In addition, SO2 reacts with other air pollutants to form sulfate particles, which are constituents of fine particulate matter (PM2.5). Inhalation exposure to PM2.5 has been associated with various cardiovascular and respiratory health effects (see PM Concentrations indicator). Many other environmental effects are associated with high concentrations of SO2. For example, airborne SO2, along with nitrogen oxides (NOx), contributes to acidic deposition (the Acid Deposition indicator); SO2 is a major precursor to PM2.5 (the PM Concentrations indicator); and SO2 contributes to impaired visibility (the Regional Haze indicator). -
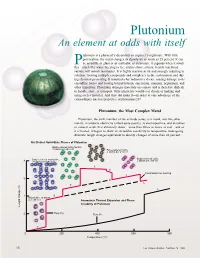
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

Reduction of Nitrogen Oxides by Carbon Monoxide Over an Iron Oxide Catalyst Under Dynamic Conditions
Applied Catalysis B: Environmental 17 (1998) 357±369 Reduction of nitrogen oxides by carbon monoxide over an iron oxide catalyst under dynamic conditions Harvey Randall, Ralf Doepper, Albert Renken* Institute of Chemical Engineering, Federal Institute of Technology, 1015 Lausanne, Switzerland Received 6 July 1997; received in revised form 23 November 1997; accepted 2 December 1997 Abstract The reduction of NO and N2O by CO over a silica-supported iron oxide catalyst was investigated by the transient response method, with different initial oxidation states of the catalyst, i.e. completely reduced (Fe3O4), or oxidised (Fe2O3). The in¯uence of CO pre-adsorption was also studied. From the material balance on the gas phase species, it was shown that the composition of the catalyst changes during relaxation to steady-state. The degree of reduction of the catalyst at steady-state could thus be estimated. During the transient period, CO was shown to inhibit N2O as well as NO reductions by adsorption on reduced sites. The activity of the reduced catalyst was found to be substantially higher as compared to the oxidised catalyst for both reactions. On this basis, it was attempted to keep the catalyst in a reduced state by periodically reducing it with CO. As a result, a signi®cant increase in the performance of the reactor with respect to steady-state operation could be achieved for N2O reduction by CO. Finally, the dynamic behaviour of the N2O±CO and NO±CO reactions made it possible to evidence reaction steps, the occurrence of which could not be shown during our previous investigations on the separate interactions of the reactants with the catalyst. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH".