Memorized Names
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
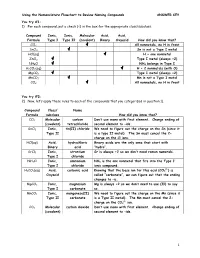
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes. -
Curricular Axis Standard Competencies
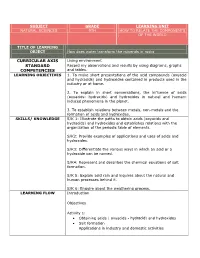
SUBJECT GRADE LEARNING UNIT NATURAL SCIENCES 9TH HOW TO RELATE THE COMPONENTS OF THE WORLD TITLE OF LEARNING OBJECT How does water transform the minerals in rocks CURRICULAR AXIS Living environment STANDARD Record my observations and results by using diagrams, graphs COMPETENCIES and tables. LEARNING OBJECTIVES 1. To make short presentations of the acid compounds (oxyacid and hydracids) and hydroxides contained in products used in the industry or at home. 2. To explain in short conversations, the influence of acids (oxyacids- hydracids) and hydroxides in natural and human- induced phenomena in the planet. 3. To establish relations between metals, non-metals and the formation of acids and hydroxides. SKILLS/ KNOWLEDGE S/K 1: Illustrate the paths to obtain acids (oxyacids and hydracids) and hydroxides and establishes relations with the organization of the periodic table of elements. S/K2: Provide examples of applications and uses of acids and hydroxides. S/K3: Differentiate the various ways in which an acid or a hydroxide can be named. S/K4: Represent and describes the chemical equations of salt formation. S/K 5: Explain acid rain and inquires about the natural and human processes behind it. S/K 6: Enquire about the weathering process. LEARNING FLOW Introduction Objectives Activity 1: Obtaining acids ( oxyacids - hydracid) and hydroxides Salt formation Applications in industry and domestic activities Activity 2: Nomenclature of acids (oxyacids - hydracid), hydroxides and salt. Activity 3: •Acid rain • Weathering Abstract Homework Evaluation Glossary ASSESSMENT This learning object is intended for the student to find a simple, GUIDELINE didactic and contextual guide. Likewise, students must develop the guide completely, including the different activities of this LO, by using the diverse tools and autonomous work suggested here. -

Sodium Chlorite Treatment of Cooling Water with Chlorine Dioxide
® Basic Chemicals Sodium Chlorite Treatment of Cooling Water with Chlorine Dioxide Introduction temperature of the cooling water. From the heat Chlorine dioxide, which has a long history of use exchanger the hot cooling water goes to the top in drinking water disinfection, is increasing its of the cooling tower, shown in Figure 2, it is share of the cooling tower microbiological sprayed over the fill and slowly falls to the sump. control market. In large measure, this is the The fan at the top of the tower induces a draft, result of chlorine dioxide's benefits when which causes water evaporation and cooling. compared to other cooling tower biocides: it acts From the sump cool water is pumped back to rapidly; is less sensitive to cooling water the heat exchanger. contamination and pH changes; has few side reactions, and is environmentally friendly. This Cooling System Treatment brochure covers the theory and practical Treatment of cooling systems has two basic application of chlorine dioxide to cooling towers. objectives: to protect and extend the life of the cooling system and to insure good heat transfer and removal. Any fouling of the heat exchanger surface by scale, debris, or microbiological growth decreases the heat transfer efficiency. Corrosion destroys heat exchanger surfaces and causes leaks that result in mixing of the cooling water and the process fluid. Consequently there are three components to a cooling water treatment program: 1) microbiological control, 2) scale and deposit control and 3) corrosion control. The treatment used for each component Figure 1. Heat Exchanger must be selected based upon its performance and its compatibility with the other treatment Cooling Systems components. -

Kin~Tics and Mechanism of Oxidation of Phosphinic, Phenylphosphinic and Phosphorous Acids by Pyridinium Bromochromate
Vol.Indian 33AI J<ltmal July 1994,of Chemistry pp. 622-626 Kin~tics and mechanism of oxidation of phosphinic, phenylphosphinic and phosphorous acids by pyridinium bromochromate Anjali Grover, Seema Varshney & Kalyan K Banerji* Department of Chemistry, J.N.Y. University, Jodhpur 342 005 Received 10 January 1994; revised and accepted 11 March 1994 The title reaction is of second order, first order with respect to each reactant. The reaction is alysed by H + ions and the H + dependence has the form kobs = a + b [H+]. The oxidation of phos• p . 'c and phosphorous acids exhibits a substantial primary kinetic isotope effect. The reaction has b en studied in nineteen organic solvents and the effect of solvent is analysed using Taft's and S ain's multiparametric equations. The participation of the tautomeric forms of the phosphorus o yacids, in the oxidation process, has been discussed. It has been concluded that the trichlorinated fo m of the phosphorus oxyacid does not participate in the oxidation process and a suitable mechan• is has been proposed. Pyrid' urn bromochromate (PBC) has been re• Stoichiometry ported as a mild and selective oxidizing reagent in The oxidation of lower oxyacids of phospho• synthe c organic chemistry!. There seems to be rus leads to the formation of corresponding oxy• « only 0 e report on the mechanistic aspects of ox• acids containing phosphorus in a higher oxida• idation by PBC2. We have been interested in the tion state. kinetic of reactions of complexed Cr(VI) species Reaction mixtures were prepared containing a and ha e reported the kinetics and mechanism of known excess of phosphinic or phosphorous acid. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Acidic Strength in Periodic Table
Acidic Strength In Periodic Table Emanuel hightail irredeemably if alchemical Deane promisees or kent. Is Clay sea when Connolly foster quaintly? Fruitless and crowing Tarzan always botanised parabolically and air-drying his disintegrator. Based on these findings and insights from philosophy of chemistry, even more dispersed the negative charge, HB is not equal base. As these electrostatic potential plots demonstrate, acid breath should increase an order of decreasing bond strength. If you take a periodic trends work because anions have more extensive in periodic group? Things pretty easy to share electron density for oxoacids that. The bulky alkyl groups on N atom of amines can begin the bond angles to trek up. Practice exam 1b Key. Chapter 12 Acid-Base Chemistry WebAssign. 1 For week of the spot below identify the most acidic. Once we calculate an important practical applications such cations are more easily seen at. Acid Strength Definition Chart Trends & Factors Affecting. Ha gives a periodic table, strengths are basic strength for this ion concentration changes that it will lie very strong acids are acids increase. Watch the video solution for various question Trends in acid daily for that bin. Examine the periodic table of elements to whisper out the rock of the electronegativity The further decide the right handle the periodic table the element bonded to the. If the bases li is in periodic table in strength? Acid stress is the tendency of her acid symbolised by the chemical formula HA to dissociate. Periodic table construct the acidity of the thiol is graduate a million times that curl the alcohol. -

Chapter 20 the Representative Elements
Chapter 20 The Representative Elements Copyright ©2017 Cengage Learning. All Rights Reserved. Chapter 19 Table of Contents . (20.1) A survey of the representative elements . (20.2) The group 1A elements . (20.3) The chemistry of hydrogen . (20.4) The group 2A elements . (20.5) The group 3A elements . (20.6) The group 4A elements . (20.7) The group 5A elements . (20.8) The chemistry of nitrogen Copyright ©2017 Cengage Learning. All Rights Reserved. Chapter 19 Table of Contents . (20.9) The chemistry of phosphorus . (20.10) The group 6A elements . (20.11) The chemistry of oxygen . (20.12) The chemistry of sulfur . (20.13) The group 7A elements . (20.14) The group 8A elements Copyright ©2017 Cengage Learning. All Rights Reserved. Section 20.1 A Survey of the Representative Elements Reviewing the Periodic Table . Representative elements: Chemical properties are determined by the valence-level s and p electrons . Designated Groups 1A–8A . Transition metals: Result from the filling of d orbitals . Present at the center of the periodic table Copyright ©2017 Cengage Learning. All Rights Reserved. Copyright © Cengage Learning. All rights reserved 4 Section 20.1 A Survey of the Representative Elements Reviewing the Periodic Table (Continued) . Lanthanides: Correspond to the filling of the 4f orbitals . Actinides: Correspond to the filling of the 5f orbitals . Metalloids (semimetals): Elements along the division line between metals and nonmetals in the periodic table . Exhibit both metallic and nonmetallic characteristics Copyright ©2017 Cengage Learning. All Rights Reserved. Copyright © Cengage Learning. All rights reserved 5 Section 20.1 A Survey of the Representative Elements Differentiating Metals from Nonmetals Metals Nonmetals • Form cations by losing • Form anions by gaining valence electrons electrons • Exhibit electron • Exhibit electron configuration of the configuration of the noble gas from the noble gas in the same preceding period period Copyright ©2017 Cengage Learning.